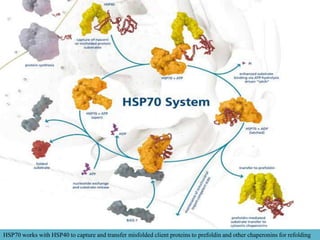
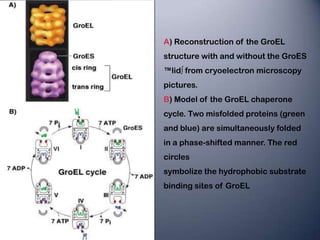
Heat shock proteins (HSPs) help maintain protein structure and prevent aggregation. They are classified by molecular weight and function. HSP100, HSP90, HSP70, HSP60 and small HSPs aid in protein folding, transport, and solubilization. When stressed, proteins can unfold but HSPs bind to prevent aggregation and allow refolding. HSPs are more stable due to strong hydrogen bonds and packing that maintain their structure even in stressful conditions.