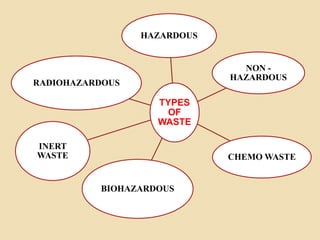
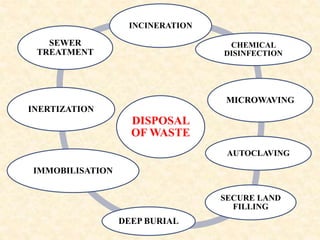
The document outlines pharmaceutical waste types and disposal methods, categorizing waste into hazardous, non-hazardous, biohazardous, and chemotherapy wastes. It details disposal techniques such as incineration, autoclaving, and chemical disinfection, emphasizing proper handling and management to minimize environmental impact. Additionally, it highlights waste management strategies including waste minimization, reuse, and recycling in compliance with health guidelines.