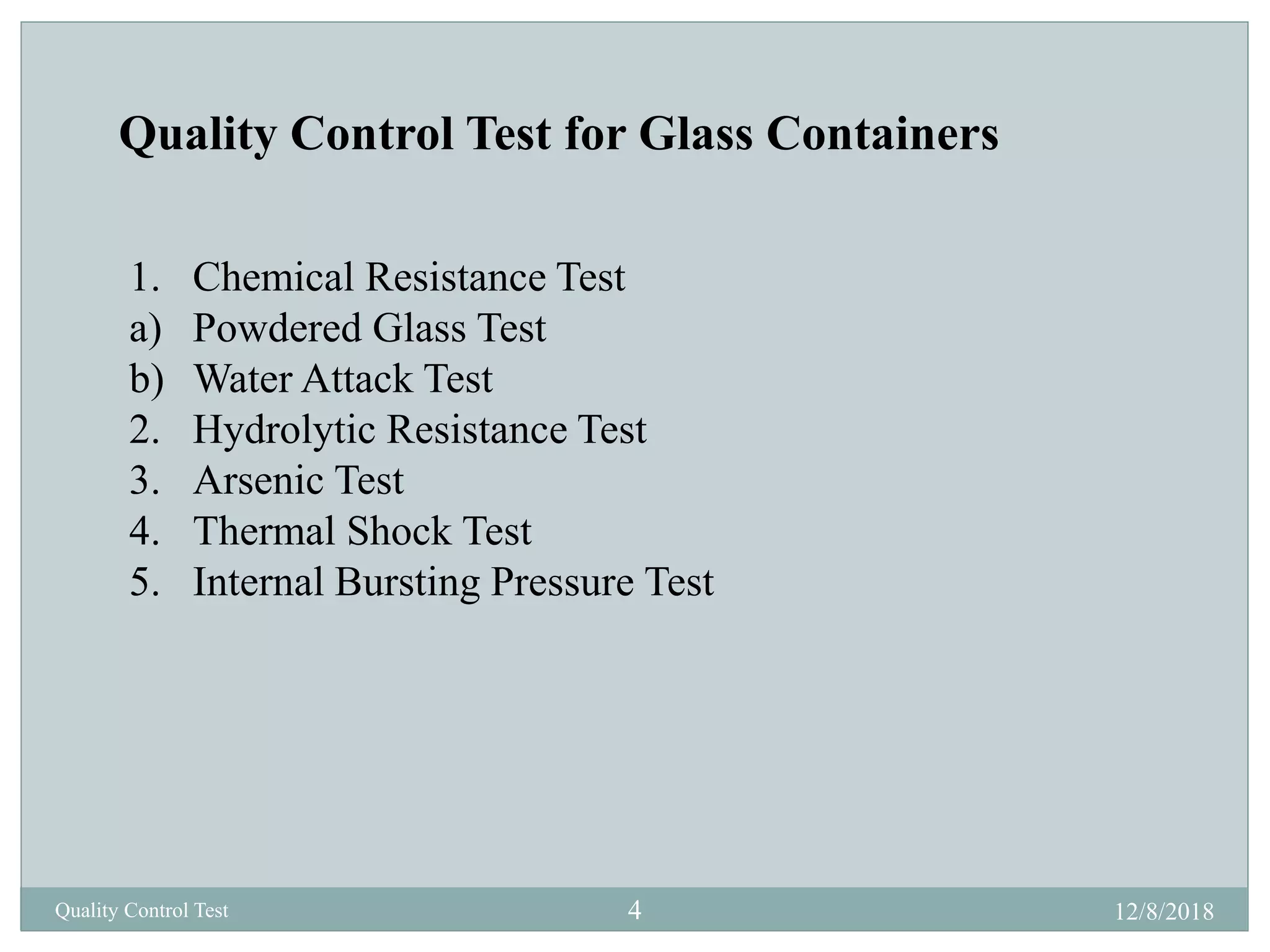
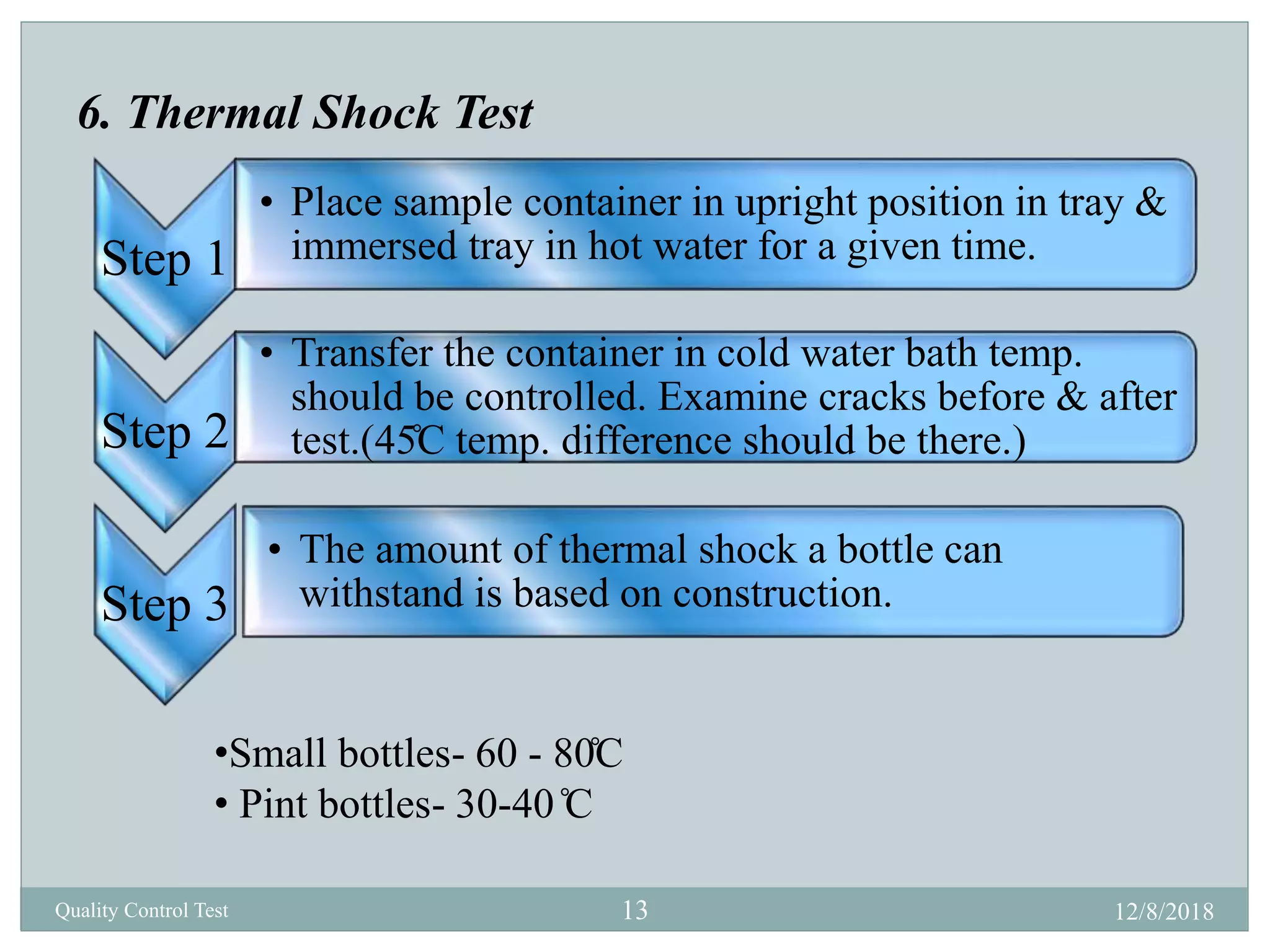
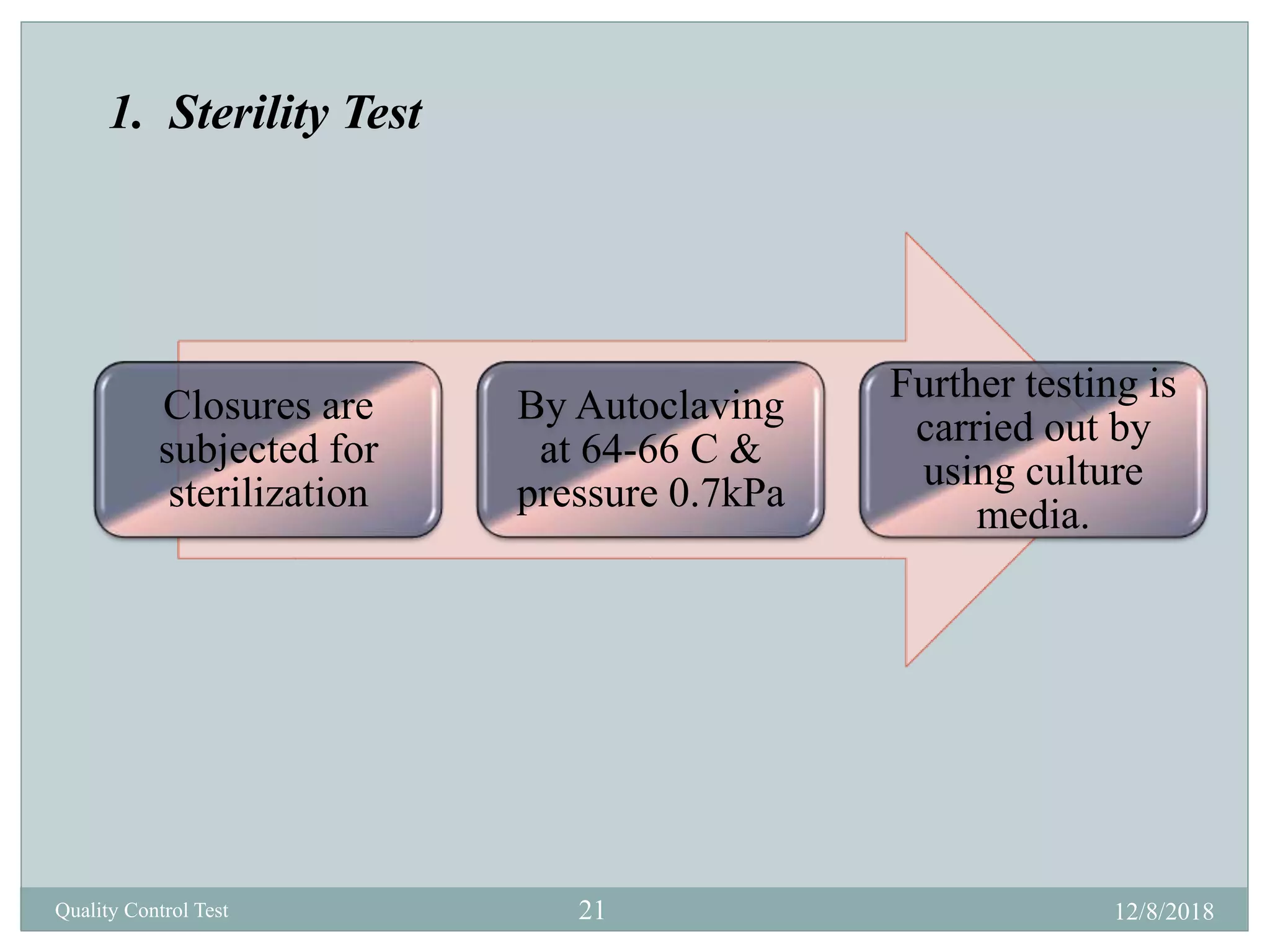
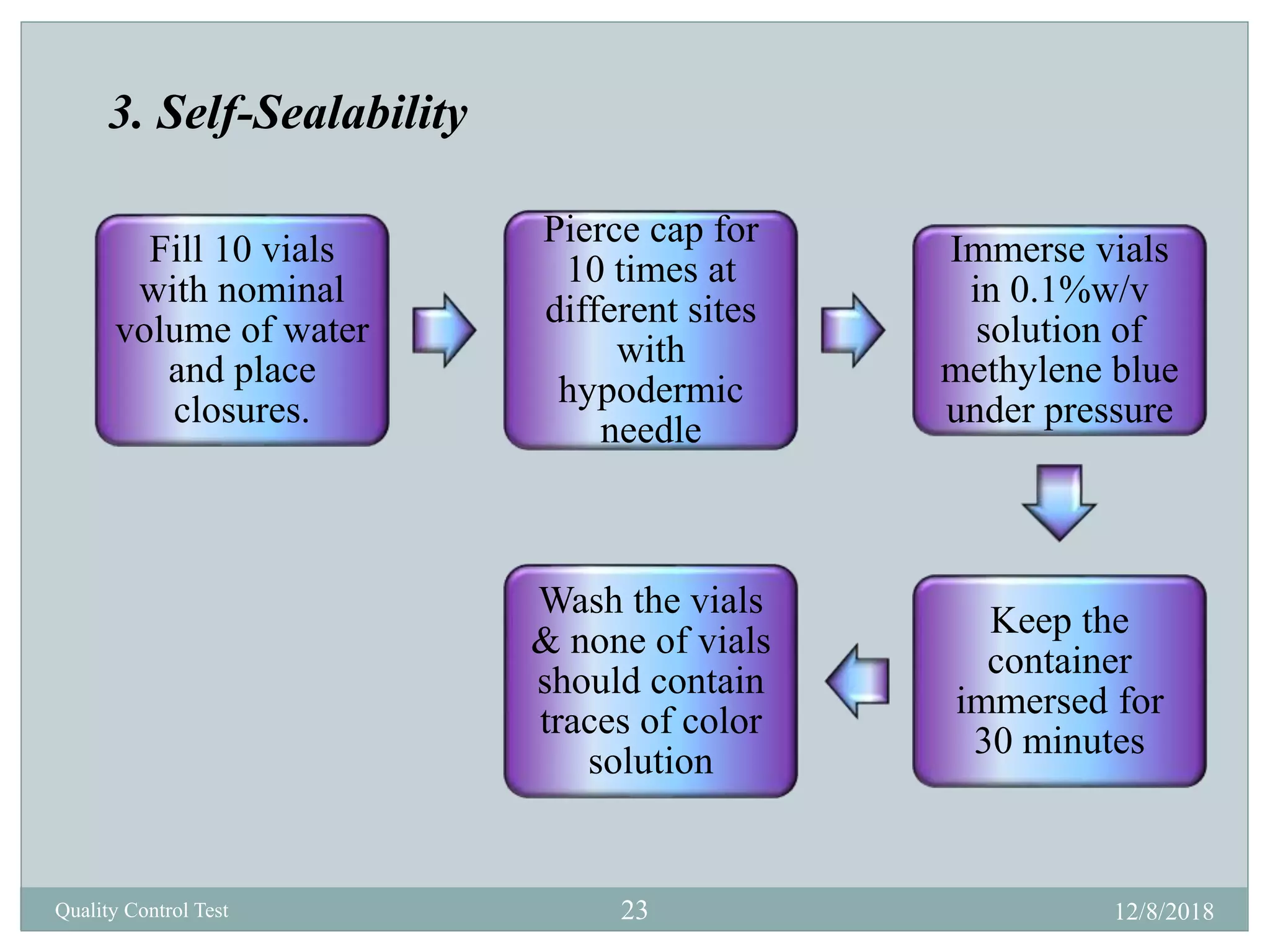
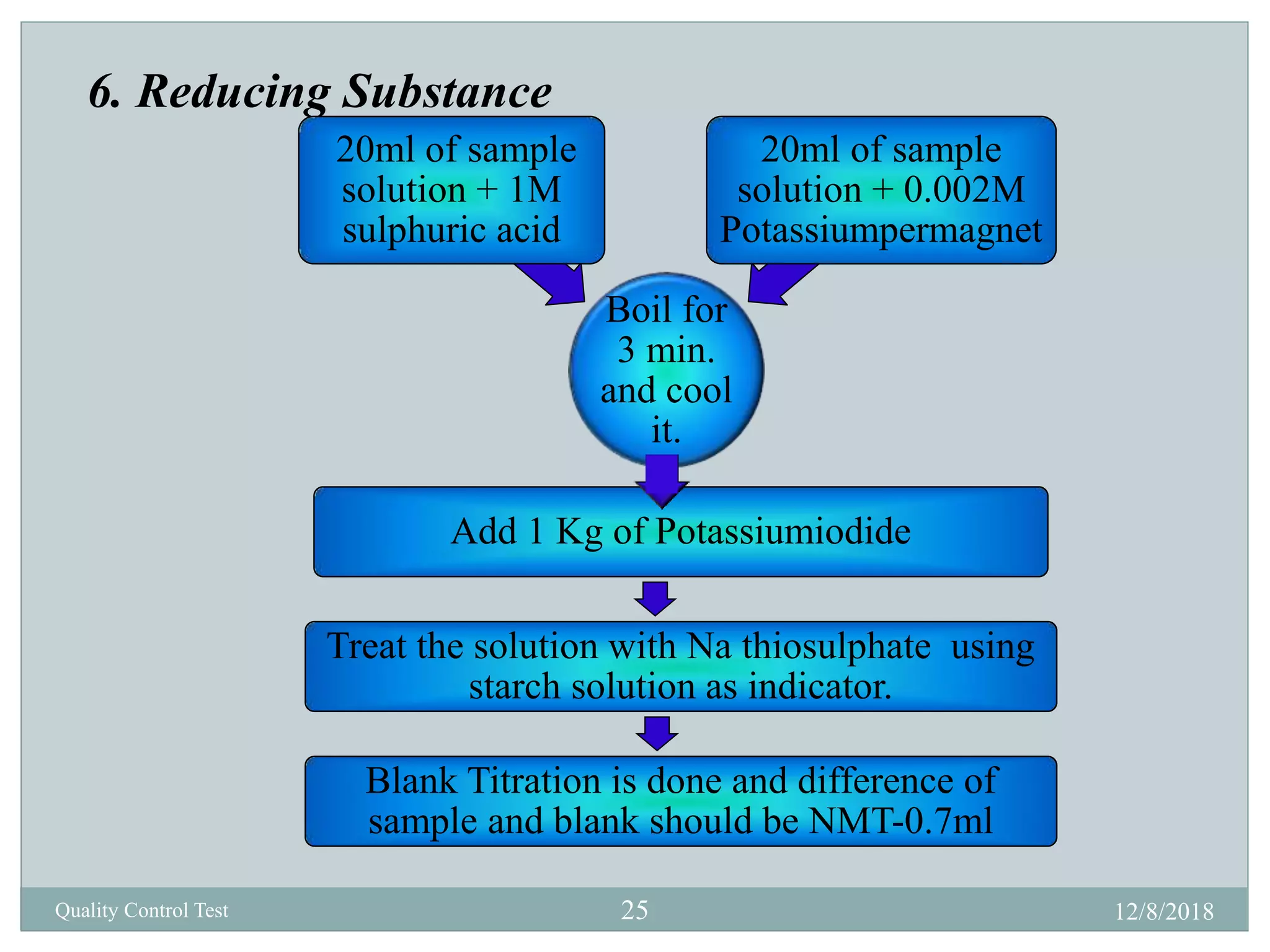
This document describes quality control tests for various pharmaceutical packaging materials including containers, closures, and secondary packaging. It provides details on tests for glass, plastic, and metal containers to evaluate properties like chemical resistance, leakage, hydrolytic resistance, and thermal shock resistance. Tests for closures examine sterility, fragmentation, self-sealability, pH, and absorption. Secondary packaging materials are tested for moisture content, folding endurance, air permeability, tensile strength, and burst resistance. The document provides testing methodologies and acceptance limits for ensuring packaging integrity and suitability for drug products.