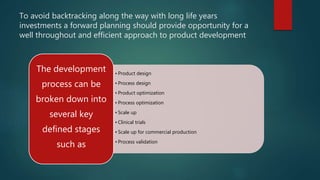
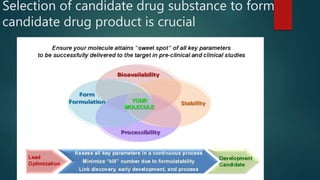
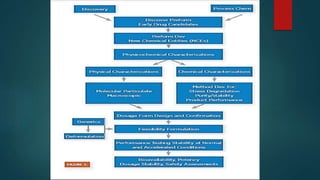
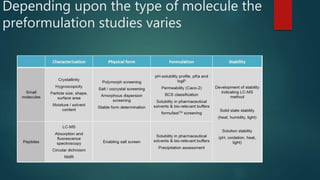
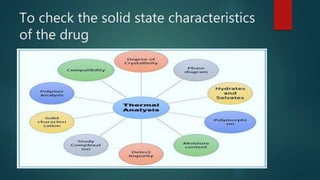
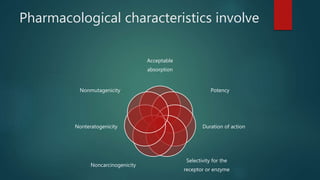
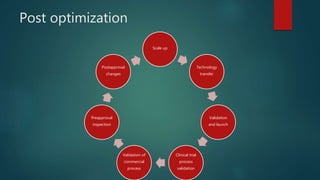
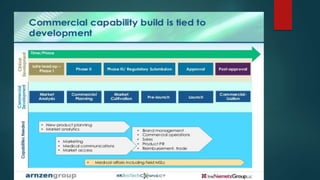
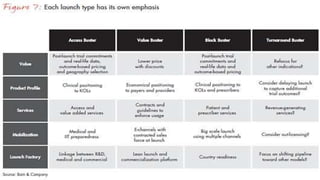
This document discusses the key stages of pharmaceutical preformulation and formulation development. It begins with an introduction and overview of the product development cycle. It then covers topics like candidate drug selection, pharmacological characterization, biopharmaceutical considerations, early drug development and product design, product optimization, and post-optimization activities. For each stage, it provides brief explanations of the goals, studies, and factors considered. The overall summary is that this document outlines the major steps involved in taking a new drug candidate through preclinical testing and formulation optimization to become a developed pharmaceutical product ready for clinical trials and commercialization.