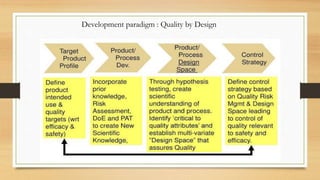
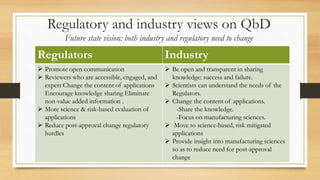
The document focuses on Quality by Design (QbD) in pharmaceutical development, which is a systematic approach ensuring products meet predefined quality objectives through process understanding and control. It outlines key characteristics of QbD, components of pharmaceutical development, and the importance of scientific approaches in regulatory submissions. Ultimately, it emphasizes a shift from traditional validation to continuous quality verification, enhancing the efficiency and safety of drug products.

















![For hyphenated techniques e.g.
i. In LC–MS method development
ii. In bioanalytical method development
• Quality-by-Design Based Development of a Self-Micro-emulsifying Drug Delivery
System [SMEDDS] to Reduce Food Effect of Nelfinavir Mesylate
• Quality by Design Approach for Optimizing the Formulation and Physical
Properties of Extemporaneously Prepared Orodispersible Films:](https://image.slidesharecdn.com/quality-by-designinpharmaceuticaldevelopment-200317130228/85/Quality-by-design-in-pharmaceutical-development-20-320.jpg)


