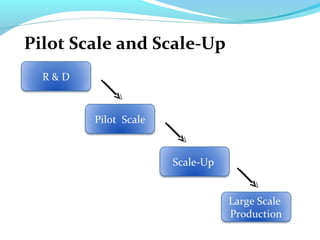
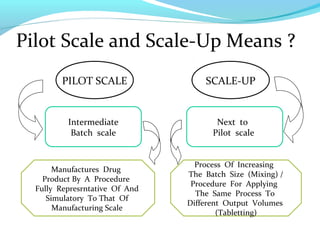
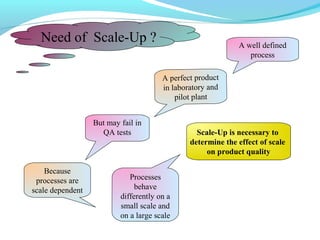
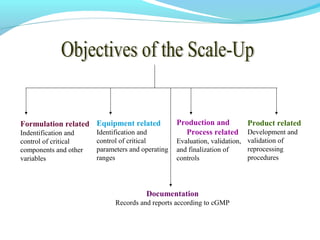
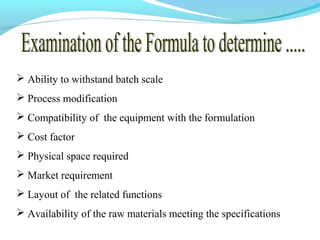
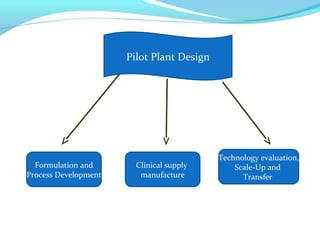
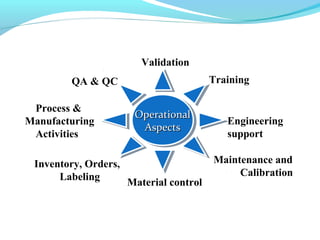
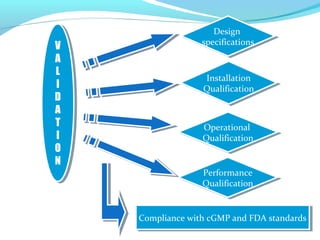
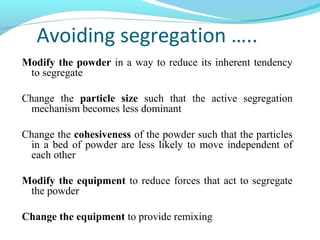
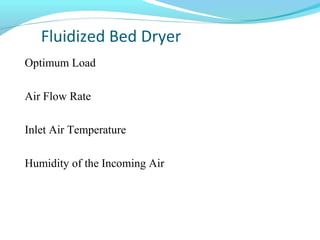
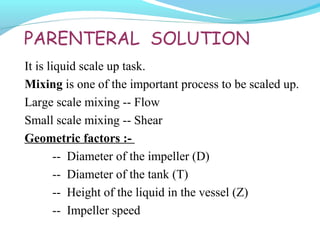
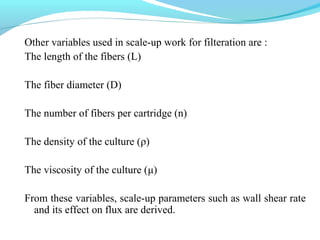
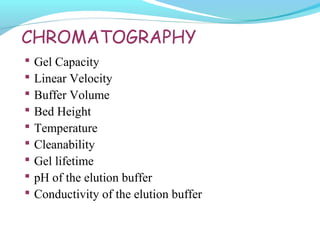
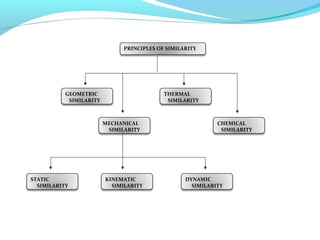
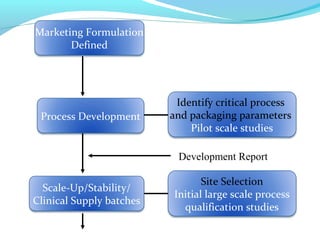
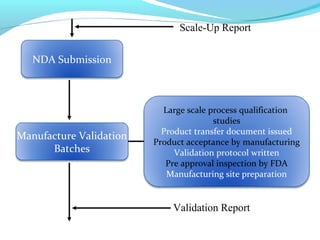
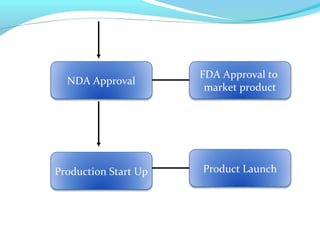
This document discusses pilot plant design, operation, and scale up considerations for pharmaceutical manufacturing processes. It provides an overview of the need for pilot plants and scale up to transfer processes from the laboratory to production scale. Key sections include descriptions of pilot plant attributes, operational aspects like validation and training, scale up principles of similarity, and development milestones from formulation through clinical and commercial production. Process parameters that should be evaluated at various stages like mixing, drying, milling and compression are also outlined.