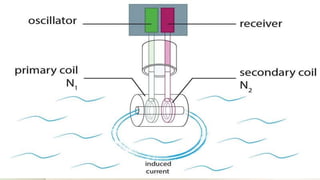
This document discusses conductivity meters, which measure the electrical conductivity of solutions. It describes two main types - contacting meters with electrodes, and inductive meters with wire coils. Conductivity depends on temperature and ion concentration, and is calibrated using standard solutions. Conductivity meters are used to monitor water quality, detect leaks, and ensure cleaning procedures in industries like pharmaceuticals.