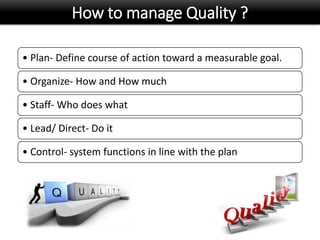
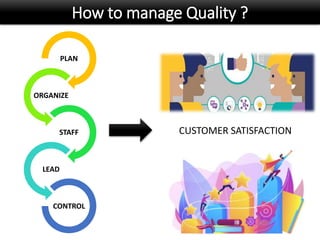
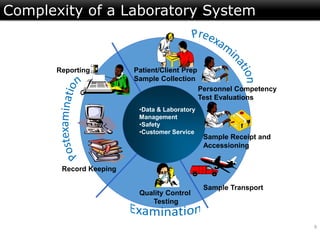
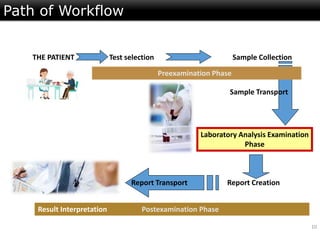
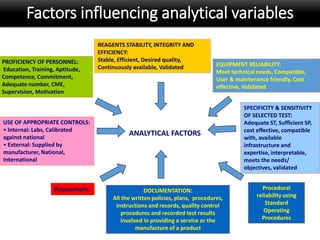
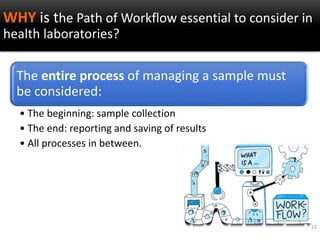
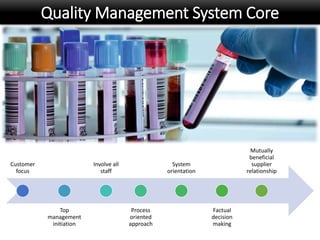
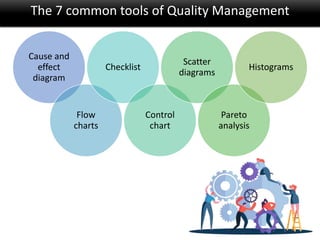
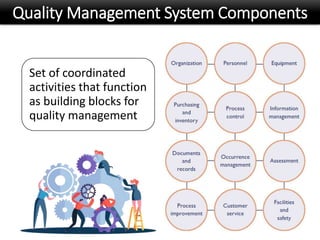
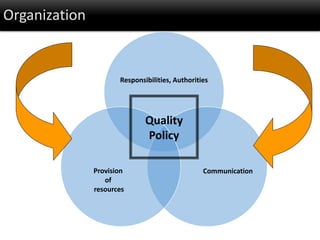
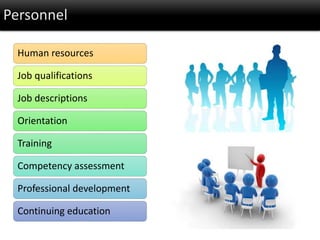
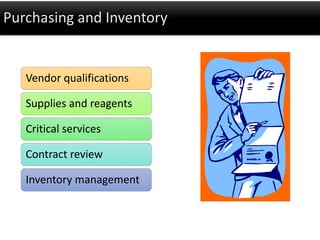
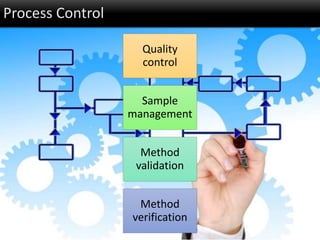
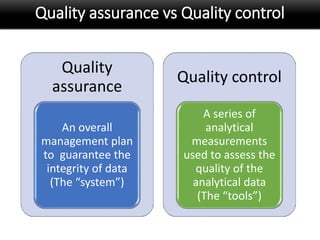
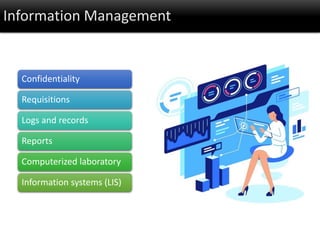
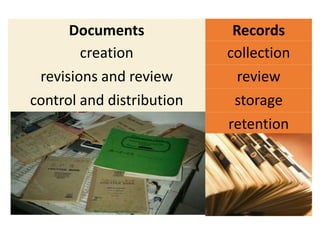
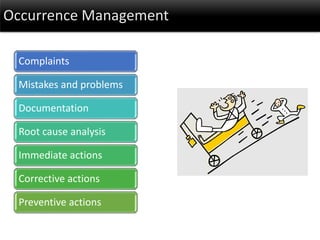
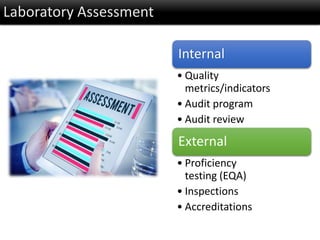
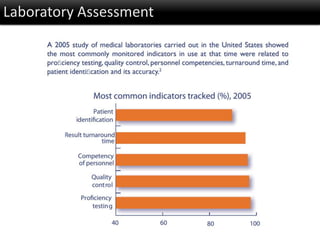
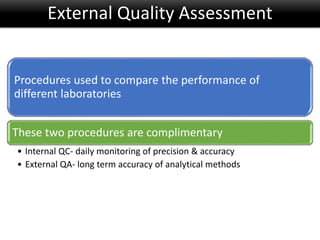
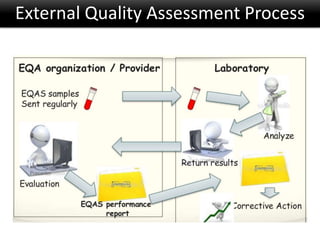
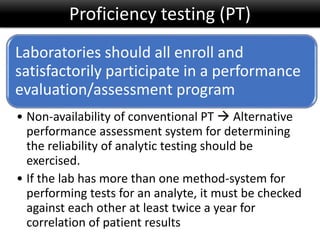
This document discusses quality management in health laboratories. It defines quality and outlines approaches to quality management including planning, organizing, staffing, leading and controlling processes. Key elements of a quality management system are described such as organization, personnel, equipment, purchasing, process control, information management, documents/records, occurrence management and assessment. The document emphasizes that a quality management system involves coordinated activities across all aspects of laboratory operations to ensure quality. External quality assessment through proficiency testing is also discussed as an important tool for evaluating laboratory performance.




![What are Quality metrics/indicators?
Established measures used to determine how well an
organization meets needs and operational and
performance expectations
• Laboratory shall implement quality indicators to
systematically monitor and evaluate its contribution to
patient care. ISO 15189 [4.12.4]
• When the programme identifies opportunities for
improvement, the laboratory management shall address
them, regardless of where they occur.
• Laboratory management shall ensure that the laboratory
participates in quality improvement activities that deal
with relevant areas and outcomes of patient care.](https://image.slidesharecdn.com/qualitymanagementsystemprasenjit-200205040149/85/Quality-Management-System-5-320.jpg)