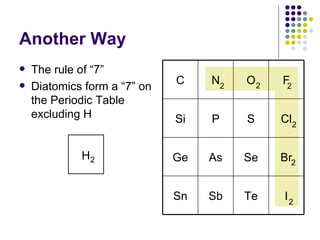
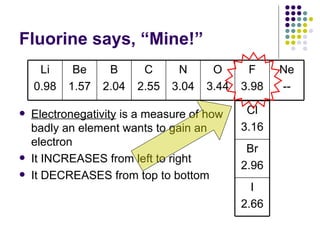
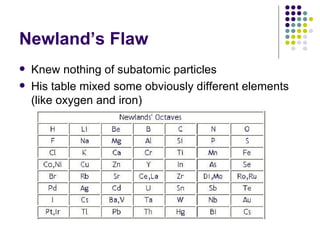
The document discusses the early development of the periodic table by several scientists in the late 19th century. John Newlands noticed a pattern in properties repeating every 8 elements in 1863 but was ridiculed. Dmitri Mendeleev produced the first recognizable periodic table in 1869, arranging elements by atomic mass and leaving gaps for undiscovered elements. Henry Moseley experimentally determined atomic number in 1914, improving upon Mendeleev's work and more clearly explaining periodic trends.






![Moseley’s Lost Nobel Many thought he should have won Nobel Prize. It’s only given to the living…he was shot in the head by a sniper in Gallipoli. Bohr (1962): "You see actually the Rutherford work [the nuclear atom] was not taken seriously. We cannot understand today, but it was not taken seriously at all. There was no mention of it any place. The great change came from Moseley." British barred scientists from enlisting for combat.](https://image.slidesharecdn.com/notes111408to112108-1228349856669063-8/85/Notes-11-14-08-To-11-21-08-9-320.jpg)
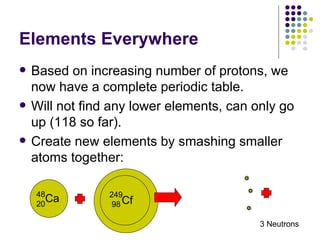









![Alkali Metals Group 1 (excluding hydrogen) [ns 1 ] Soft, lustrous, oxidize when exposed to air. Difficult to isolate – never found in nature. React (violently) with water to form a base. React with chlorine to form a salt with a 1-to-1 ratio: LiCl NaCl KCl RbCl CsCl (also FrCl)](https://image.slidesharecdn.com/notes111408to112108-1228349856669063-8/85/Notes-11-14-08-To-11-21-08-22-320.jpg)
![Alkaline Earth Metals Group 2 [ns 2 ] Harder & Denser than Alkali Metals. Lustrous, oxidize slowly when exposed to air. React with water or steam to form a base. React with chlorine to form a salt with a 1-to-2 ratio: BeCl 2 MgCl 2 CaCl 2 SrCl 2 BaCl 2 RaCl 2](https://image.slidesharecdn.com/notes111408to112108-1228349856669063-8/85/Notes-11-14-08-To-11-21-08-23-320.jpg)
![Halogens Group 17 [np 5 ] Nonmetals Gases (F, Cl), liquid (Br), and solids (I, At) Name means “salt former.” React with sodium to form a salt with a 1-to-1 ratio: NaF NaCl NaBr NaI NaAt](https://image.slidesharecdn.com/notes111408to112108-1228349856669063-8/85/Notes-11-14-08-To-11-21-08-24-320.jpg)
![Noble Gases Group 18 [np 6 ] Unreactive Gases – colorless, odorless. Some of the last natural elements to be discovered. Once called “Inert Gases.” Monatomic in Nature](https://image.slidesharecdn.com/notes111408to112108-1228349856669063-8/85/Notes-11-14-08-To-11-21-08-25-320.jpg)

![Transition Metals Groups 3 to 12 [nd x ] Central portion of the PT. Behavior and appearance vary. Variable oxidation state (charge). Different oxidation states can produce different colors. Often used to make pigments. Co +2 Cr +6 Cr +6 Ni +2 Cu +2 Mn +7](https://image.slidesharecdn.com/notes111408to112108-1228349856669063-8/85/Notes-11-14-08-To-11-21-08-27-320.jpg)
![Lanthanoids 1 st Row on Bottom of table [4f x ] AKA Lanthanides & Rare Earths Not so rare (Ce 25 th most abundant) So similar, very difficult to separate – remember Moseley? Most deflect UV – used in sunglasses Shiny, silvery white, soft, react violently with most nonmetals, tarnish in air](https://image.slidesharecdn.com/notes111408to112108-1228349856669063-8/85/Notes-11-14-08-To-11-21-08-28-320.jpg)
![Actinoids 2 nd Row on Bottom of table [5f x ] AKA Actinides All are radioactive Not as similar as the Lanthanoids Only Th and U are common in nature Most are man-made Nuclear fallout Particle colliders](https://image.slidesharecdn.com/notes111408to112108-1228349856669063-8/85/Notes-11-14-08-To-11-21-08-29-320.jpg)