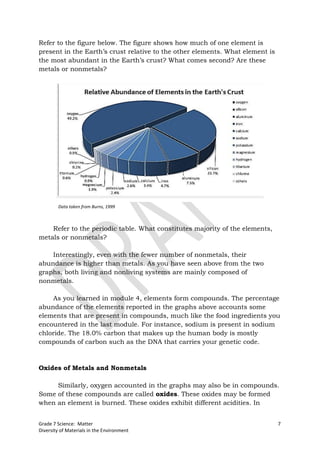
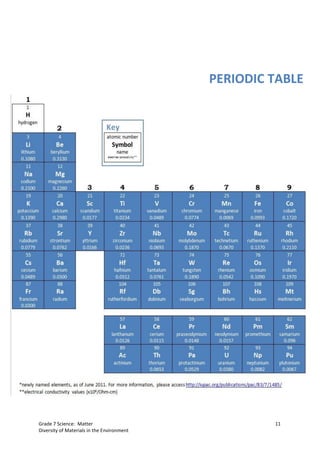
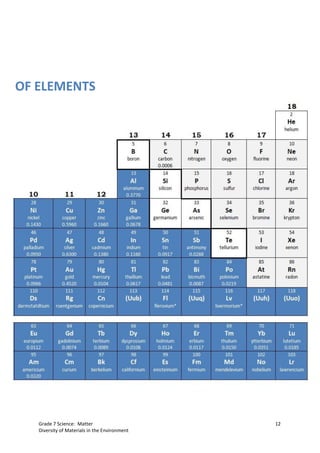
The document discusses the properties and classification of metals and nonmetals. It describes how metals are electrically conductive while most nonmetals are not, with carbon being an exception. Activities are presented to test samples for electrical conductivity and to determine if oxides of metals or nonmetals are acidic or basic. The document concludes that understanding material properties allows for designing objects with optimal functions by combining materials like copper and rubber in electrical wires.