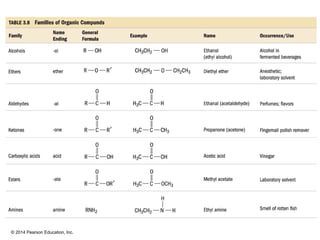
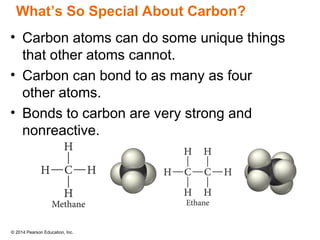
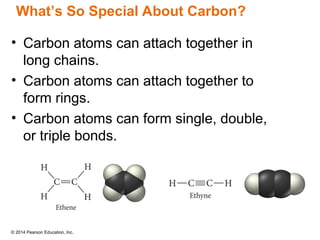
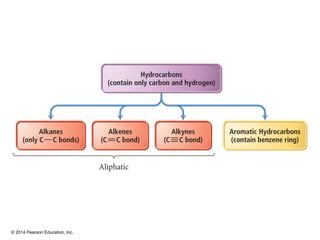
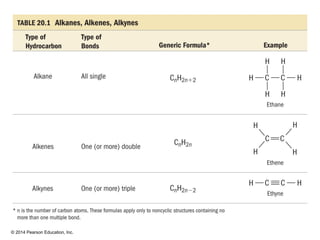
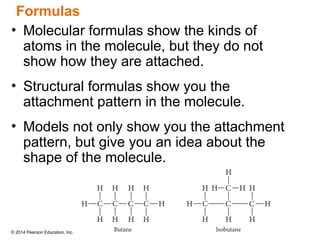
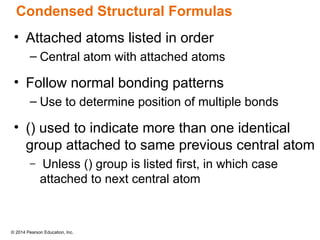
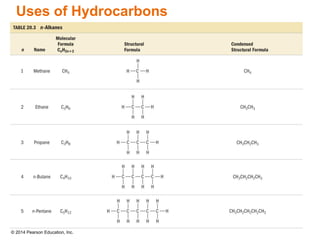
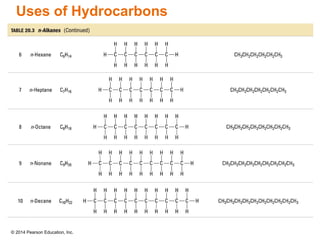
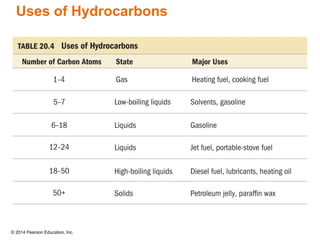
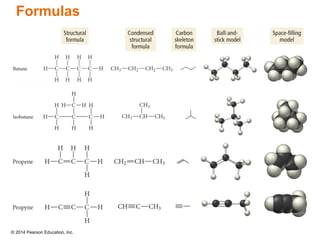
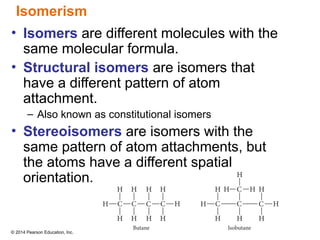
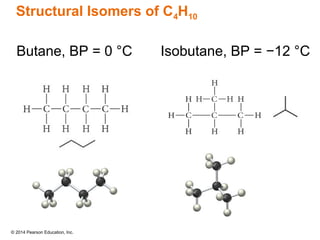
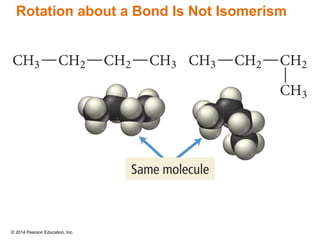
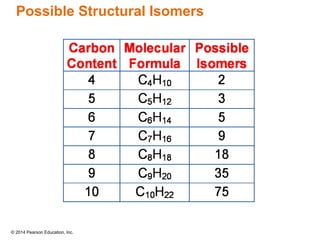
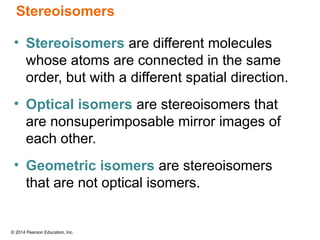
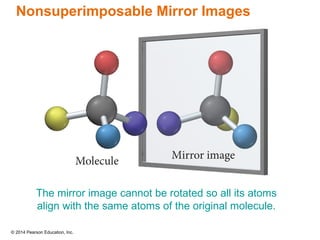
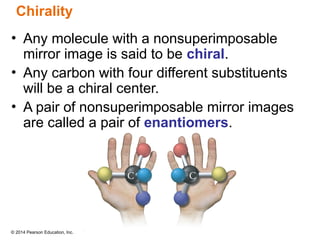
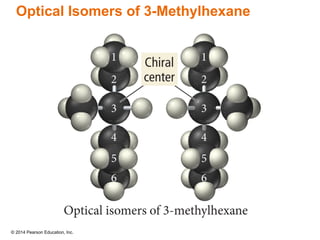
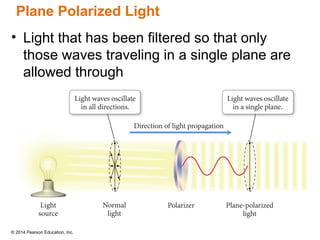
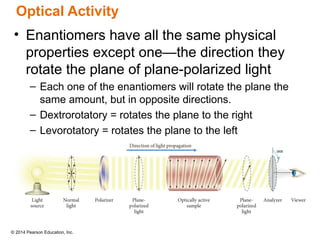
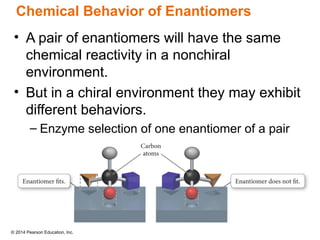
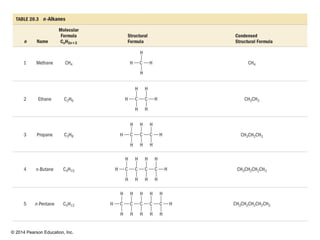
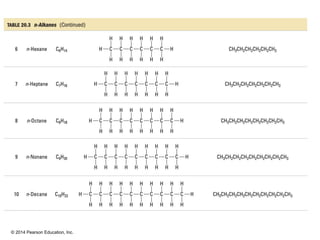
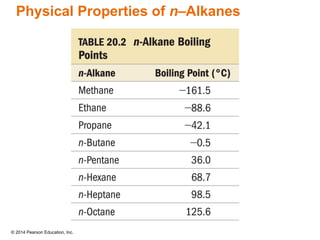
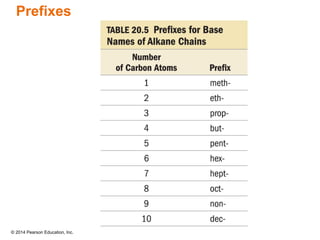
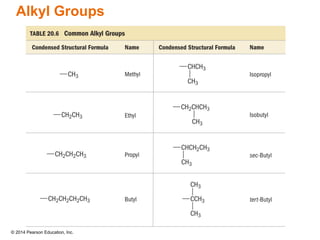
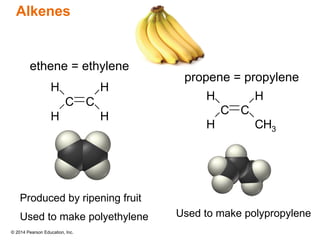
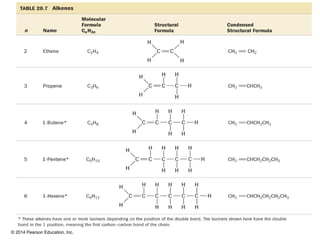
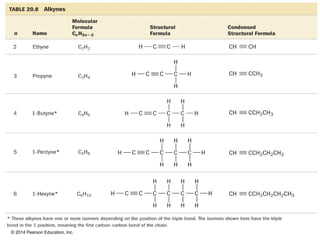
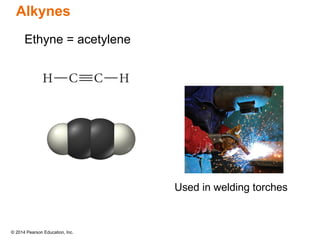
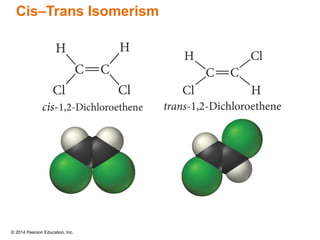
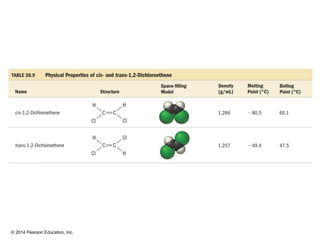
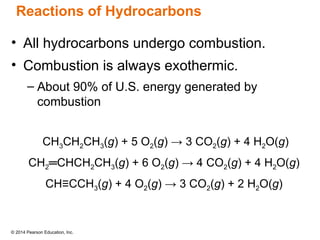
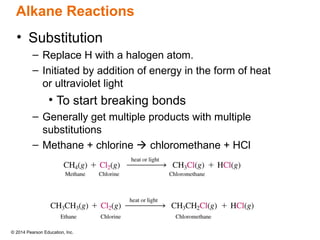
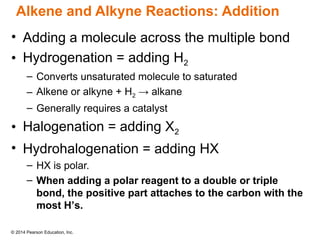
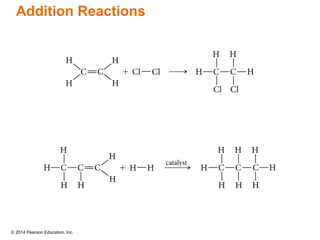
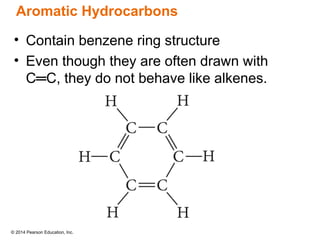
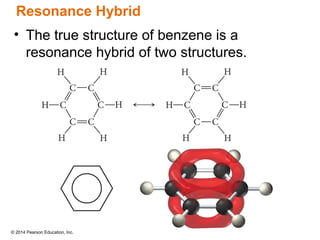
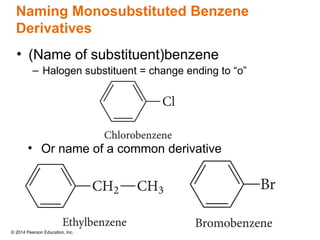
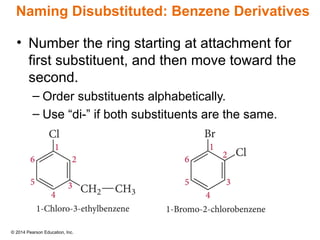
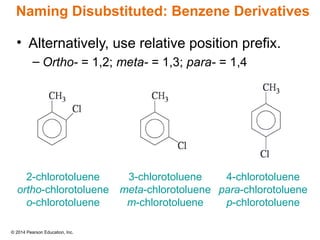
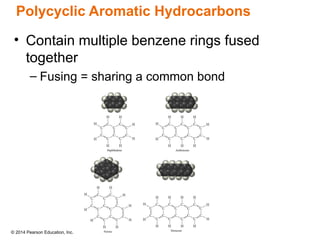
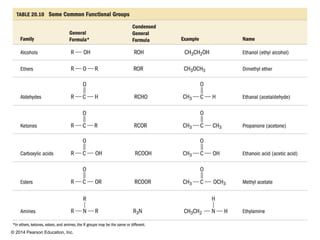
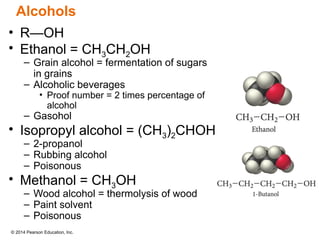
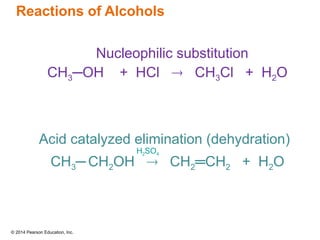
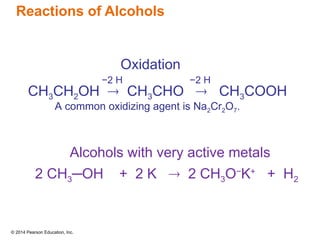
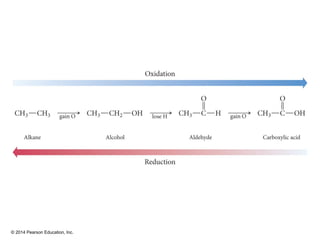
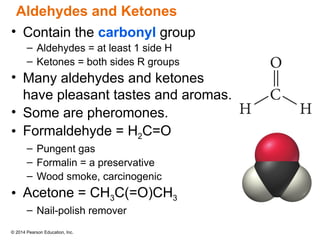
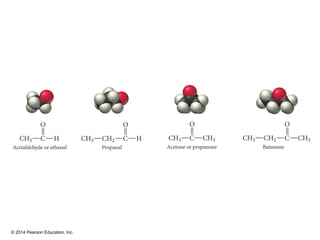
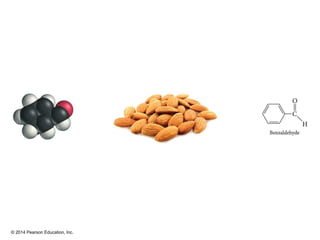
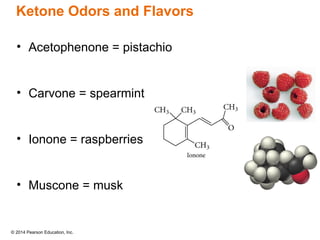
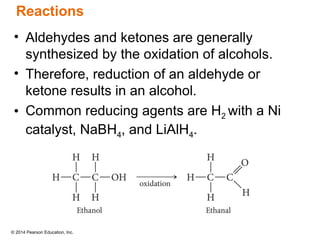
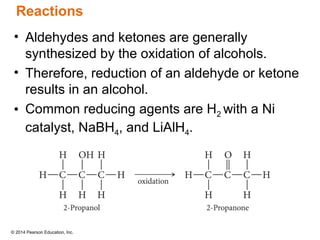
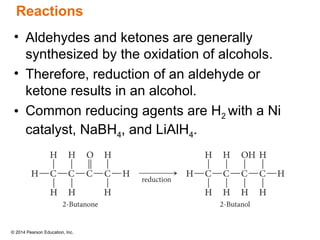
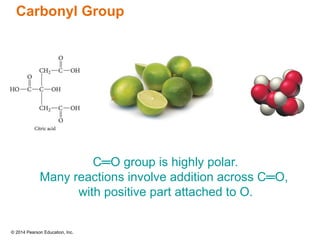
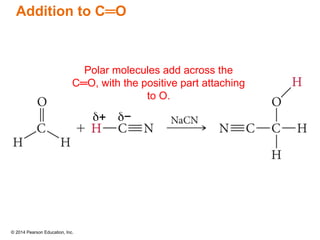
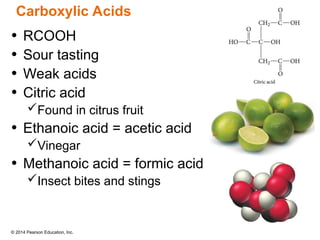
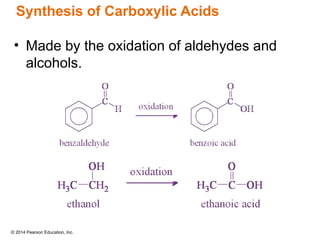
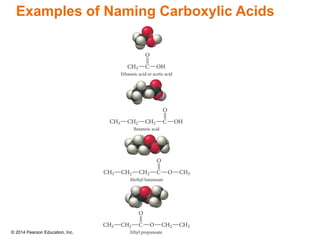
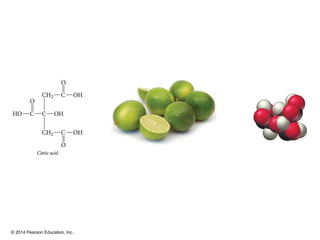
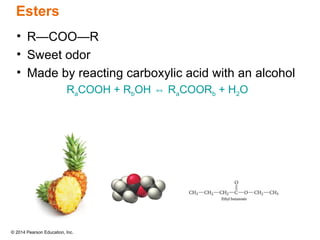
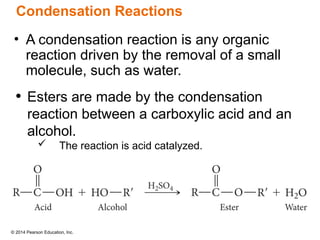
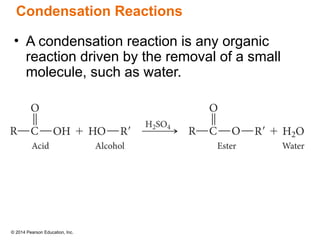
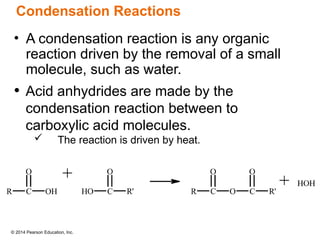
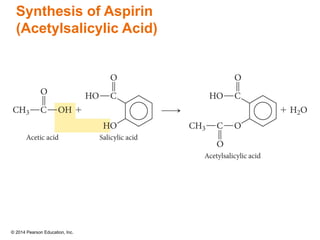
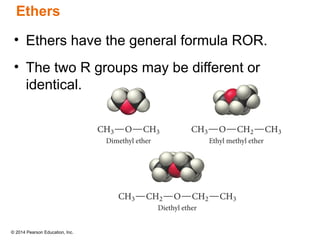
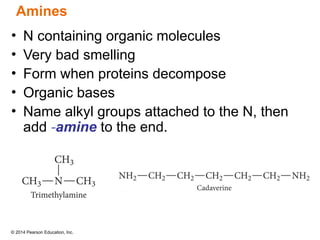
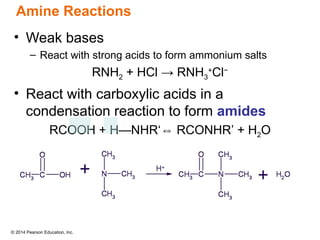
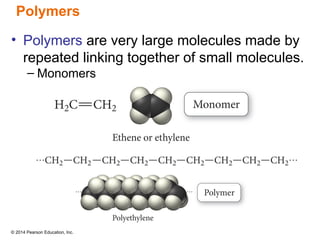
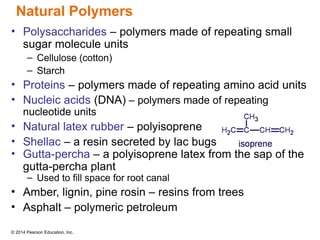
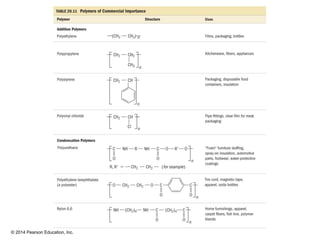
The document discusses organic chemistry, focusing on the characteristics and significance of organic compounds, particularly hydrocarbons and their properties. It explains the structural diversity of carbon compounds, isomerism, the differences between organic and inorganic substances, and the naming conventions for various hydrocarbons. Additionally, it covers specific reactions of hydrocarbons, including combustion and addition reactions involving alkenes and alkynes.