Option C, Solar Energy, Biofuel and Electron Conjugation
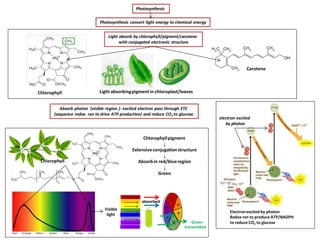
- 1. Photosynthesis convert light energy to chemical energy Photosynthesis Light absorb by chlorophyll/pigment/carotene with conjugated electronic structure Chlorophyll Carotene Light absorbingpigmentin chloroplast/leaves Visible light Green transmitted absorbed Chlorophyll Electronexcited by photon Redox rxn to produce ATP/NADPH to reduce CO2 to glucose Chlorophyllpigment ↓ Extensiveconjugationstructure ↓ Absorbin red/blueregion ↓ Green electron excited by photon Absorb photon (visible region )- excited electron pass through ETC (sequence redox rxn to drive ATP production) and reduce CO2 to glucose.
- 2. Photosynthesis convert light energy to chemical energy Photosynthesis Light absorb by chlorophyll/pigment/carotene with conjugated electronic structure Chlorophyll Carotene Light absorbingpigmentin chloroplast/leaves Absorb photon (visible region )- excited electron pass through ETC (sequence redox rxn to drive ATP production) and reduce CO2 to glucose. Electronexcited by photon Redox rxn to produce ATP/NADPH to reduce CO2 to glucose Two half eqn Eqn photosynthesis Photolysis water (Oxidation) CO2 reduction (Reduction) 6CO2 + 24H+ + 24e- → C6H12O6 + 6 H2O12H2O → 6O2 + 24H+ + 24e- 6CO2 + 6 H2O → C6H12O6 + 6O2 electron excited by photon
- 3. Triglyceride Energy for vegetable oil Too viscous Ester of fatty acid and glycerol Through biological process,agricultureand anaerobic digestion Biofuelmadefrom sugar, starch, or vegetable oil Fermentation – using sugar/corn/cane produce ethanol Biogas breakdown organic matter by anaerobic bacteria Energy Advantage Renewable Higheroctane rating Ethanol,methane – biofuel Biofuel Bioethanol C6H12O6 → C2H5OH+ 2CO2 Biogas C6H12O6 → 3CH4 + 3CO2 Biodiesel Methane Biogas - methane Disadvantage Biomassused for fuel not for food Use fertilizers, greenhousegas produced Lower specific energythan fossil fuel Transesterification – with ethanol/methanol– produce oil less viscous Strong acid/base add Reversible – smaller molecules – don’t pack – ethyl/methyl ester Methanol Ethanol Ethyl ester Methyl ester VS H+/OH- Shorterchain – less viscous
- 4. Advantages and disadvantage of biodiesel/biofuel Biomassused for fuel not for food Use fertilizers,greenhouse gas produced Lower specific energythan fossil fuel More viscous than diesel Advantage Disadvantage Renewable Carbon neutral/lowcarbon footprint Biodegradable/non toxic Higherflash pt/less flammable Higheroctane rating Ethanol,methane – biofuel Deduce equation Pentyloctanoate with methanol in presence catalyst Transesterification Produce less viscous ester Pentyl gp replace by methyl gp C7H15COOC5H11 + CH3OH → C7H15COOCH3 + C5H11OH Pentyl gp Methyl gp State eqn for complete combustion ethanol Enthalpy combustion ethanol is 1367kJ mol-1 Find specific energy in kJ g-1 Compare octane and explain diff C2H5OH + 3O2 → 3H2O + 2CO2 1mol – 1367kJ RMM ethanol = 46.08 46.08 g – 1367 kJ 1g - (1367/46.08) kJ = 29.67kJ g -1 2C8H18 + 25O2 → 8H2O + 16CO2 114.26 g – 5470 kJ 1g - (5470/114.26) kJ 1mol – 5470kJ RMM octane = 114.26 = 47.87 kJ g -1 Less energyfrom ethanol Ethanolpartiallyoxidized with OH gps attached Energy Released Ethanol < Octane State two form biomass which can be convert to energy Why biomass is likely to be impt fuel for future When biomass decompose in absence O2 , name the gas released Production of biogas, bioethanol/ biodiesel/fermentation Fossil fuel non renewable. Biomass is renewable source. Methane gas
- 5. C C Absorption of UV by organic molecule and chromophores Absorption UV radiation by C = C, C = O, N = N, N =O gps C = C /N = N (π bond) C = O: (lone pair electron) NO2 (lone pair electron) Chromophores gp Ground Higheremptyorbital π electron AbsorbUV to excite π/lone pair e to higheremptyorbital C O lone pair electron : Chromophores – organic molecule with conjugated double bond Absorb radiation to excite delocalized e to empty orbital alternating double/single bond Filled orbital Bondingorbital empty orbital antibonding orbital Biological Pigments (Anthocyanins) Coloured – extensive conjugation of electrons alternating single and double bond Porphyrin Chlorophyll Heme (hemoglobin) Anthocyanin Carotene absorb absorb absorb absorb
- 6. C C Absorption UV radiation by C = C, C = O, N = N, N =O gps C = C /N = N (π bond) C = O: (lone pair electron) NO2 (lone pair electron) Ground π electron Absorb UV to excite π/lone pair e to higheremptyorbital C O lone pair electron : alternating double/single bond Carotene Diff bet UV and Visible absorption Colourless- Absorption in UV range Electronic transitionfrom bonding to antibonding orbital (involve pi / lone pair e) UV visible Organic molecules/chromophores BiologicalPigments(Anthocyanins) Coloured – extensive conjugationofelectron Alternatingsingle and double bond Electron in pi orbitaldelocalized through single and double bond. π elec excitedby absorbinglongwavelengthin visible region Anthocyanin Chlorophyll absorb absorb Higheremptyorbital Chromophore λ max/nm C = C 175 C = O 190 C = C – C = C 210 - NO2 270 190- 260 Benzene ring – conjugatedsystem
- 7. Absorb radiation to excite delocalized e to empty orbital Filled orbital empty orbital Carotene Colourless – Absorption in UV range Electronic transition frombondingto antibondingorbital (involve pi / lonepair e) UV visible Anthocyanin Absorption of UV/vis by organic molecule and pigment Less conjugatedsystem ↓ Less alternating single/double bond ↓ Absorbshorter wavelength (UV) ↓ Colourless compound More conjugated system ↓ More alternatingsingle/double bond ↓ Absorblonger wavelength (visible) ↓ Colour compound alternating double/single bond More conjugation → More delocalization → Absorption in visible range Extensive conjugation of double bond allow more delocalization of π elec More conjugation → More delocalization → Less energy to excite electron → ↓ E lower ( absorb at visible region (colour ) How number of conjugation led to colour formation from UV to visible? BiologicalPigments(Anthocyanins) Coloured – extensiveconjugation ofelectron Alternatingsingle and double bond Electron in pi orbitaldelocalized through single and double bond. π elec excitedby absorbing long wavelength in visible region
- 8. UV visible Absorption of UV/vis by organic molecule and pigment More conjugation → More delocalization → Absorption in visible range Extensive conjugation of double bond allow more delocalization of π electron More conjugation → More delocalization → Less energy to excite electron → ↓ E lower ( absorb visible region (colour ) How number of conjugation led to colour formation from UV to visible? More conjugation – splittingenergy less ∆E ↓ – wavelengthincrease (visible range) Filled orbital empty orbital 100 200 300 400 700nmWavelength λ C – C C = C C = C – C = C C = C – C = C – C = C ∆E ↓with more conjugation absorb from UV to visible ∆E ↓with more conjugation Absorb at ↓ lower energy (↑ longer λ) AbsorbUV – sunblock Absorb visible region – food dye (Azo dye)Acid/baseindicator
- 9. alternating double/single bond CaroteneAnthocyanin Chlorophyll Heme (hemoglobin) Wavelength - absorbed Visible light Colour seen RED – RED reflect to eyes - Blue absorb (complementary colour) absorbed RED transmitted Carotenoidsabsorb λ at 460 nm Colour – extensiveconjugation ofelec.Alternatingsingle/doublebond π elec delocalizedthrough single/double bond. π elec excitedby absorbinglongwavelength in visible region 700 600 500 400 Biological Pigment
- 10. alternating double/single bond CaroteneAnthocyanin Chlorophyll Heme (hemoglobin) Wavelength - absorbed Visible light Colour seen GREEN– GREEN reflect to eyes - Red/Blue absorb (complementary colour) absorbed Green transmitted Chlorophyll absorb λ at 400 and 700nm Colour – extensiveconjugation ofelec.Alternatingsingle/doublebond π elec delocalizedthrough single/double bond. π elec excitedby absorbinglongwavelength in visible region 700 600 500 400 Biological Pigment
- 11. C6H5–(CH=CH)6–C6H5 ↓ More conjugate ↓ Absorb blue ↓ Complement colour reflect Orange C6H5–(CH=CH)5–C6H5 ↓ Less conjugate ↓ Absorbpurple ↓ Complement colour reflect Yellow Anthocyanins – used as acid/baseindicator Identify λ max which correspondto max absorbanceat diff pH and suggest colour in acid/basecondition. pH Max Colour absorb Colour pigment 1 550 Green Red 12 475 Blue Yellow/orange wavelength wavelength Anthocyanins – used as acid/base indicator Identify λ max which correspond to max absorbanceat diff pH and suggest colour in acid/base condition. pH Max Colour absorb Colour pigment 1 550 Green Red 7 350 None visible Colourless Describe relationship bet n and λ max Suggest which series absorb in visible region Suggest colour of C6H5–(CH=CH)5–C6H5 andC6H5–(CH=CH)6–C6H5 Increasen or conjugation → Absorption to longerwavelength λmax increase Absorption from 400 – 700nm ( visible region) when n > 4 n = 5 n = 6
- 12. Tetracene - Greater delocalization elec (Higher conjugation bond) - Absorb longer wavelength – visible light (colour) Organic compoundsshown anthracene and tetracene. Predict with reference to conjugation double bond,which absorbvisible light (colour) Carotene absorb light in blue/green region, so complementary colour (red and orange) are transmitted Anthracene Tetracene Absorptionspectrum ofcarotenewas shown. Explainwhy carotenehave colour. Carotene 700 600 500 400 RED Absorption spectrum of anthrocyaninis shown. Explainwhat effect,the absorptionat 375 and 530 nm have on colour of anthrocyanin At 375 nm - No effect, lies outside visible spectrum (UV region) At 530 nm - Visible colour, red, complementary to blue-green - Absorb green – Reflect Red 700 600 500 400 300 200 Anthocyanin RED
- 13. CaroteneAnthocyanin Chlorophyll Heme (hemoglobin) Wavelength - absorbed Colour seen RED – RED reflect to eye - Blue absorb Anthrocyanin – acid base indicator - absorb λ 550nm at pH 1 (acid) Colour seen Yellow – yellow reflect to eye - Blue absorb Wavelength - absorbed Anthrocyanin– acid base indicator - absorb λ 470nm at pH 12 (alkali) + H+ + OH- Add acid Add base Change in numberOH gp Changein numberconjugation Absorbat diff wavelength RED YELLOW Numberconjugation increase ↓ Absorblonger wavelength Numberconjugation decrease ↓ Absorbshorter wavelength Colour – extensiveconjugation ofelec.Alternatingsingle/double bond π elec delocalizedthroughsingle/doublebond. π elec excitedby absorbinglongwavelength in visible region Biological Pigment
