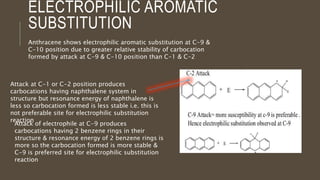
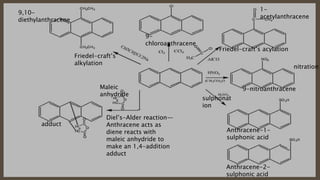
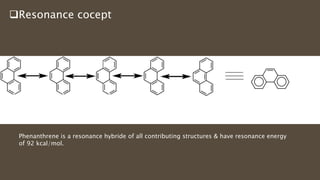
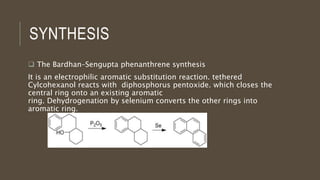
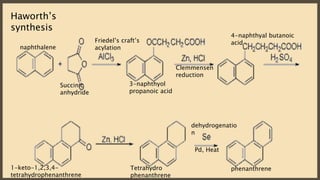
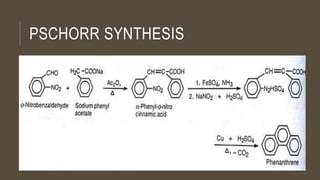
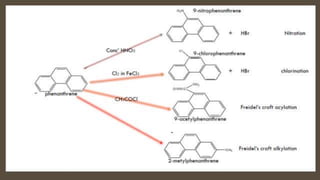
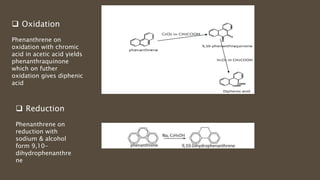
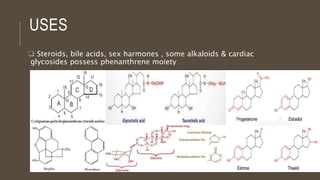
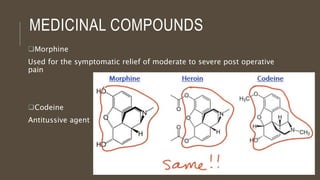
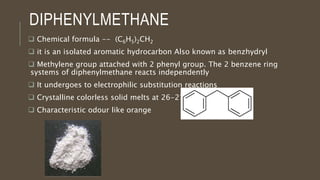
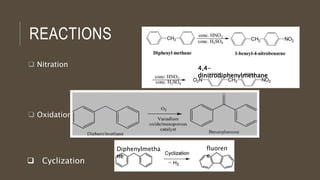
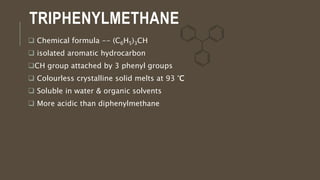
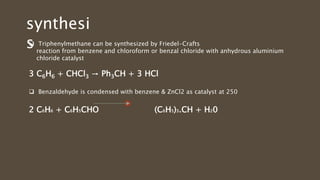
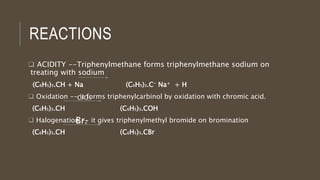
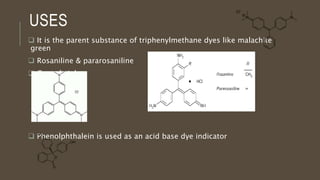
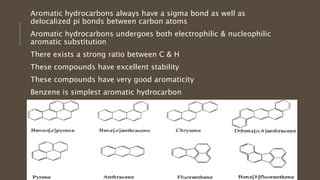
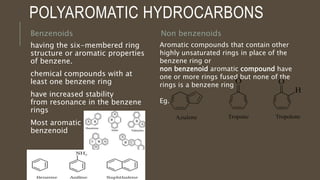
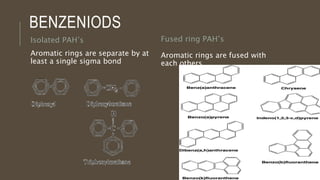
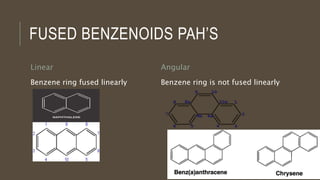
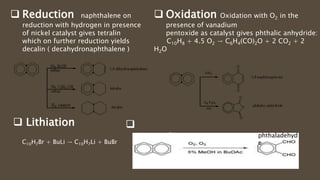
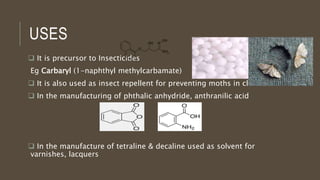
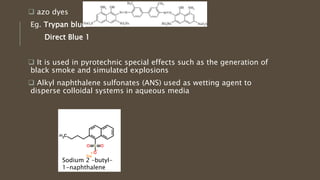
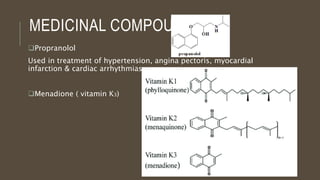
Poly-nuclear hydrocarbons are organic compounds containing multiple aromatic rings made of carbon and hydrogen. Naphthalene is the simplest poly-nuclear hydrocarbon containing two fused benzene rings. It undergoes addition and substitution reactions more easily than benzene due to its slightly lower stability. Anthracene contains three fused benzene rings and phenanthrene contains three angularly fused rings. These compounds exhibit aromatic properties and undergo electrophilic substitution. Diphenylmethane and triphenylmethane contain isolated benzene rings connected by methylene groups. These compounds find use in dyes, polymers, and pharmaceuticals.







![CYCLOADDITIONS
2 molecules of
anthracene are
connected by a pair of
new carbon-carbon
bond resulting
dianthracene
Anthracene also reacts
with dienophile singlet
oxygen in a [4+2]-
cycloaddition (Diels–
Alder reaction)](https://image.slidesharecdn.com/polycyclicaromatichydrocarbons-200705055321/85/Polycyclic-aromatic-hydrocarbons-23-320.jpg)