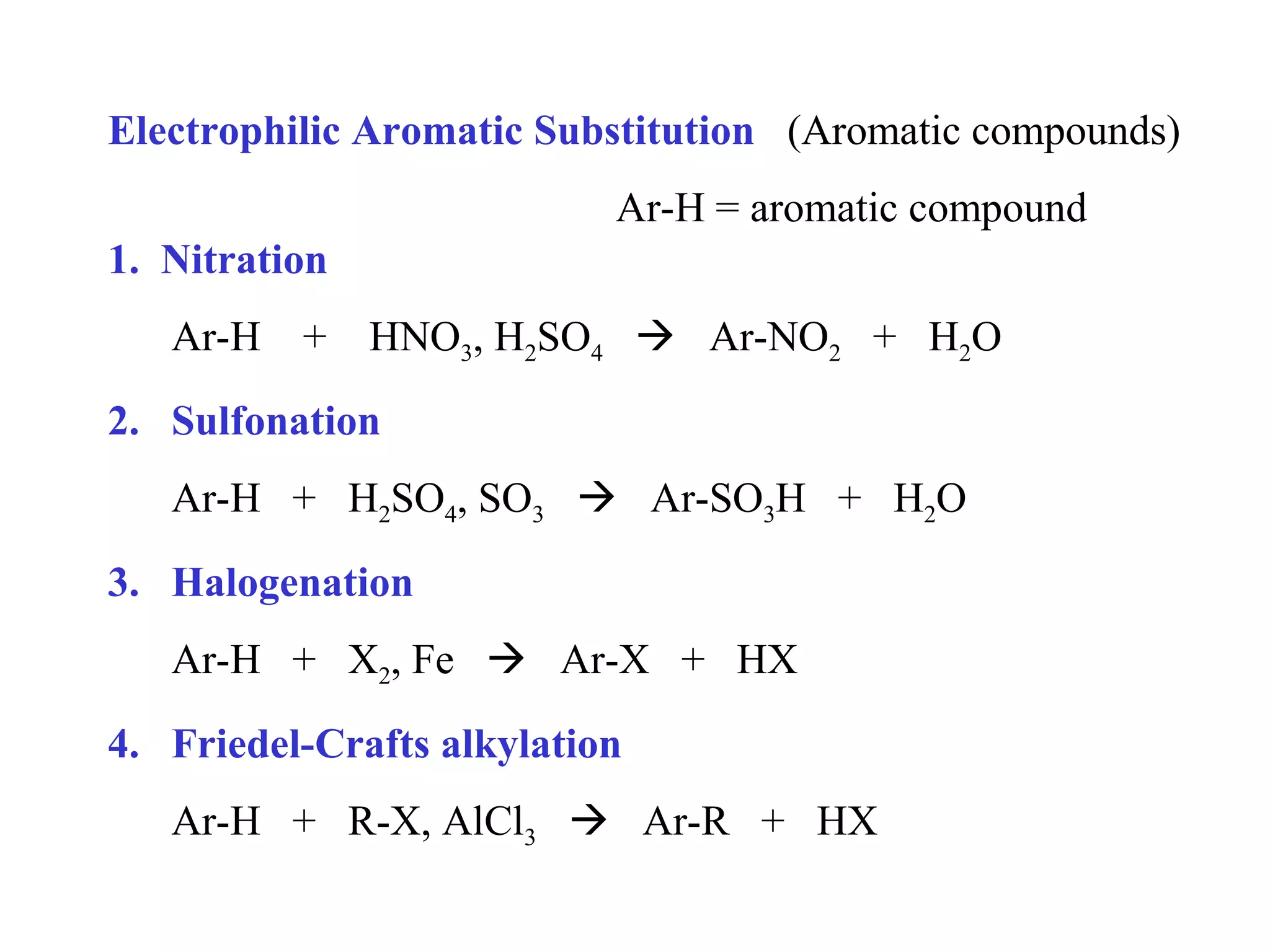
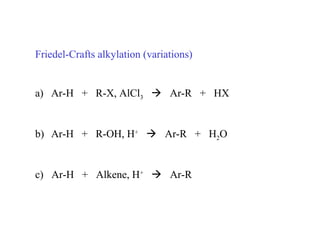
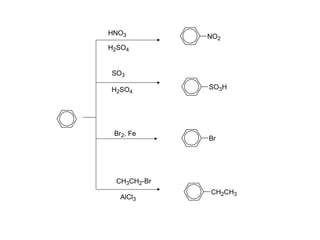
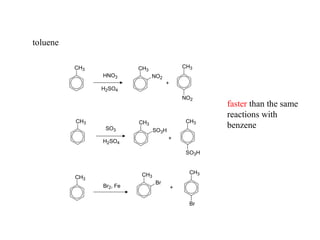
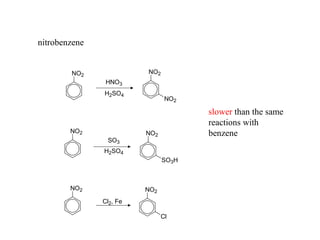
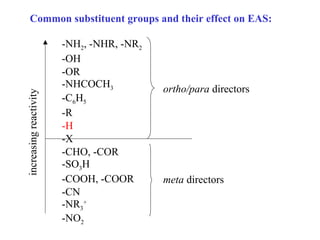
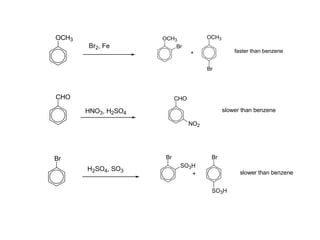
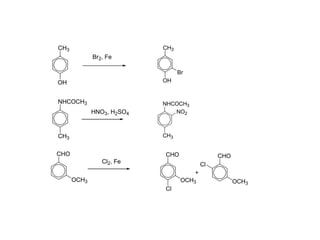
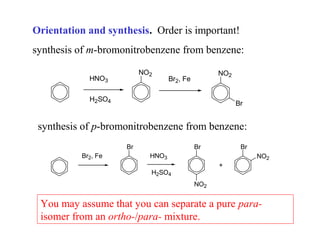
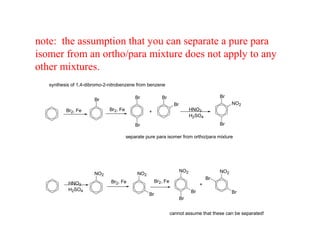
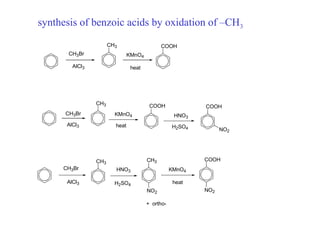
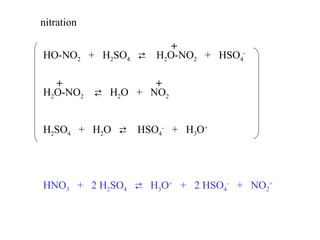
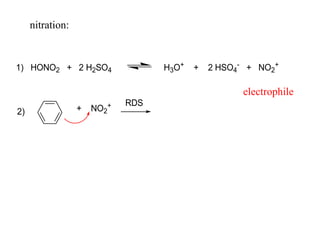
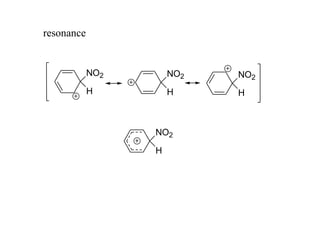
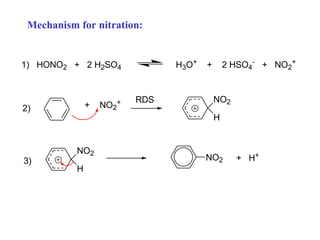
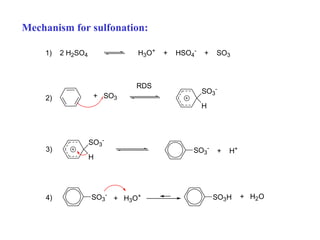
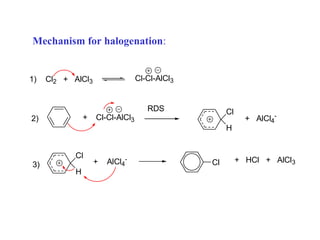
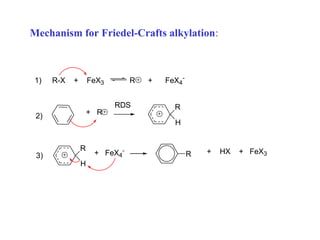
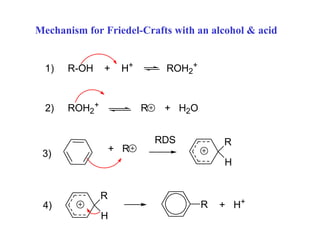
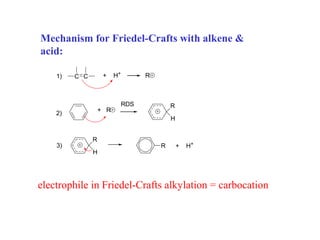
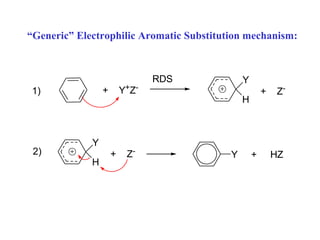
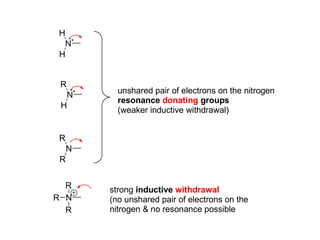
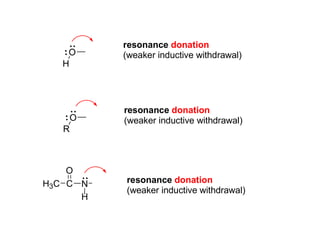
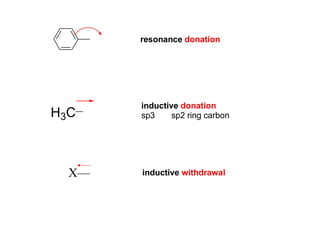
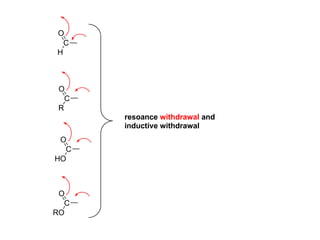
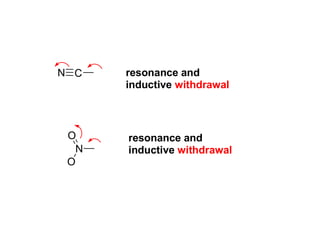
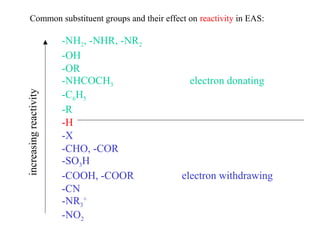
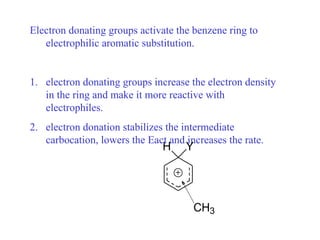
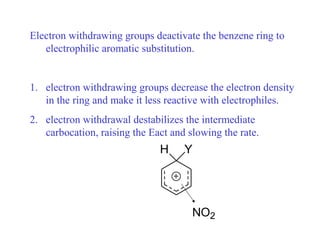
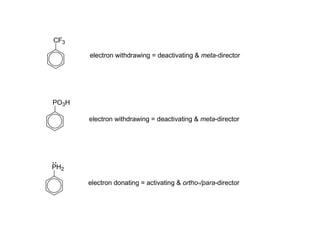
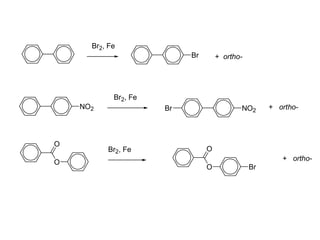
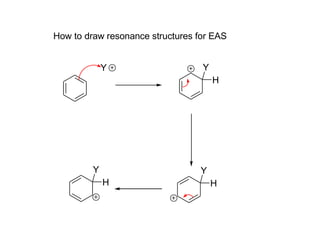
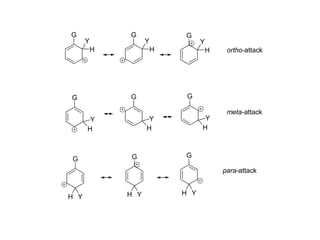
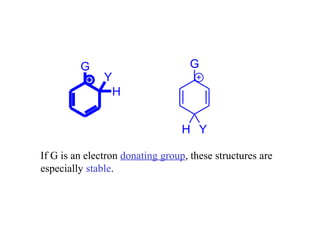
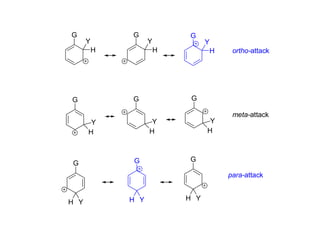
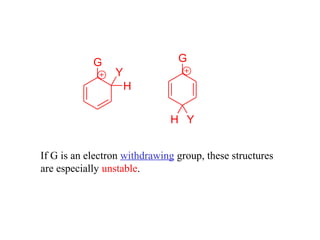
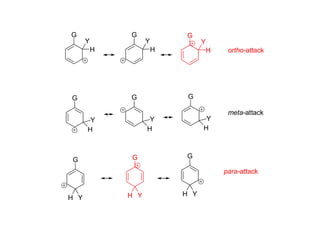
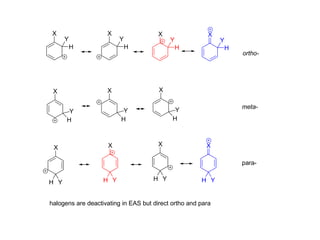
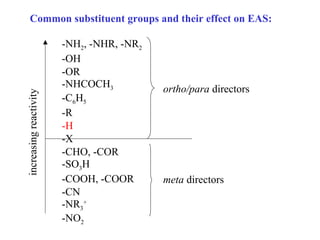
The document discusses electrophilic aromatic substitution (EAS) reactions for aromatic compounds, detailing various methods such as nitration, sulfonation, halogenation, and Friedel-Crafts alkylation, along with their mechanisms. It emphasizes the influence of substituent groups on the reactivity and orientation of these reactions, identifying which groups activate or deactivate the benzene ring and their directing effects. The document also covers resonance and inductive effects on the stability of intermediates during EAS and provides examples of reaction pathways for synthesizing specific aromatic compounds.