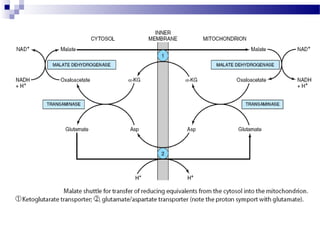
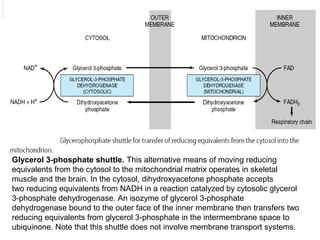
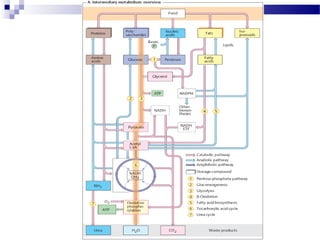
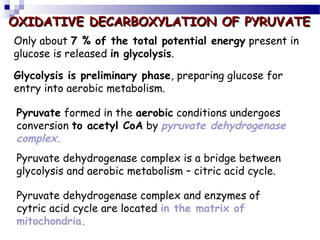
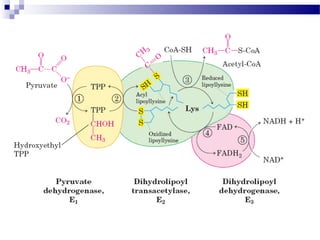
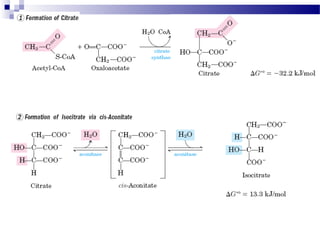
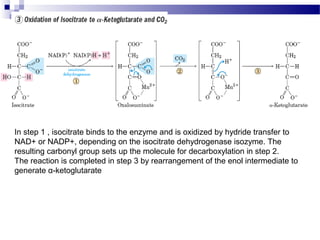
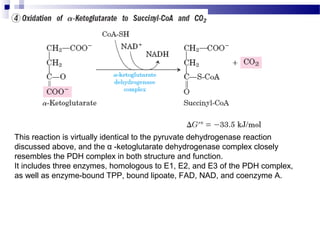
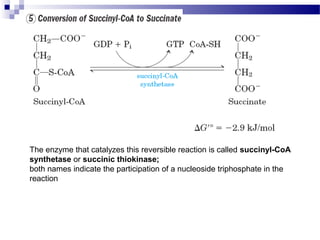
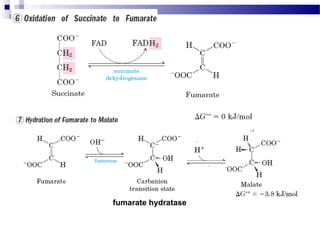
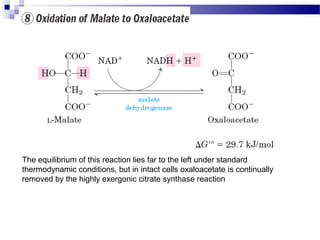
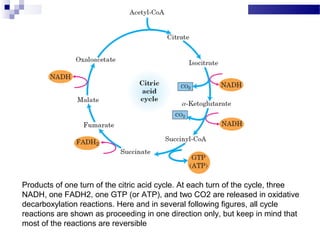
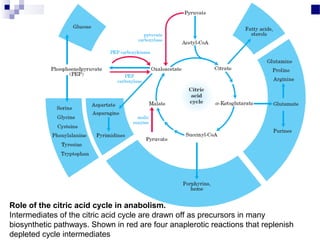
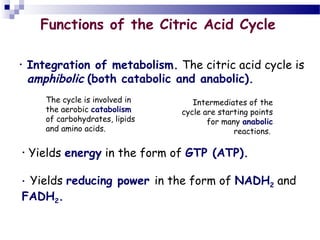
The citric acid cycle is the second stage of aerobic respiration after glycolysis. Pyruvate from glycolysis enters the mitochondrion and is converted to acetyl-CoA by the pyruvate dehydrogenase complex. Acetyl-CoA then enters the citric acid cycle where it is oxidized, releasing carbon dioxide and producing reduced coenzymes like NADH and FADH2 that will be used to generate ATP. The citric acid cycle consists of 8 steps where citrate is regenerated at the end of each cycle to continue the process. The cycle plays an important role in energy production and supplying precursors for biosynthesis.

![Recall that the conversion of one glucose molecule
to CO2 via the glycolytic pathway and citric acid
cycle yields 10 NADH and 2 FADH2 molecules.
Oxidation of these reduced coenzymes has a total
ΔG°′ of −613 kcal/mol [10(−52.6) + 2(−43.4)].
Thus, of the potential free energy present in the
chemical bonds of glucose (−680 kcal/mol), about
90 percent is conserved in the reduced coenzymes.](https://image.slidesharecdn.com/crebscycle2013-131202101225-phpapp01/85/Crebs-cycle-2013-16-320.jpg)
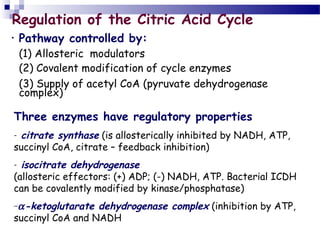
![Regulation of metabolite flow from the
PDH complex through the citric acid
cycle.
The PDH complex is allosterically inhibited
when [ATP]/[ADP], [NADH]/[NAD], and
[acetyl-CoA]/[CoA] ratios are high,
indicating an energy-sufficient metabolic
state. When these ratios decrease,
allosteric activation of pyruvate oxidation
results. The rate of flow through the citric
acid cycle can be limited by the availability
of the citrate synthase substrates,
oxaloacetate and acetyl-CoA, or of NAD,
which is depleted by its conversion to
NADH, slowing the three NAD-dependent
oxidation steps. Feedback inhibition by
succinyl-CoA, citrate, and ATP also slows
the cycle by inhibiting early steps. In
muscle tissue, Ca2 signals contraction and,
as shown here, stimulates energy-yielding
metabolism to replace the ATP consumed
by contraction.](https://image.slidesharecdn.com/crebscycle2013-131202101225-phpapp01/85/Crebs-cycle-2013-20-320.jpg)