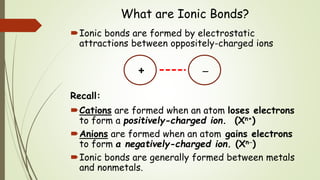
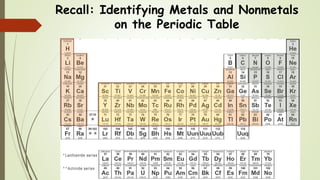
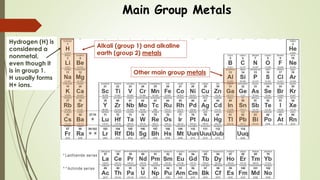
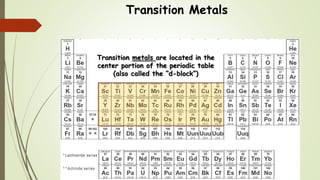
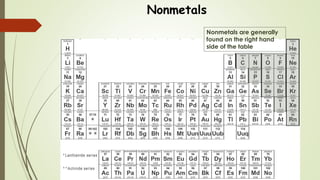
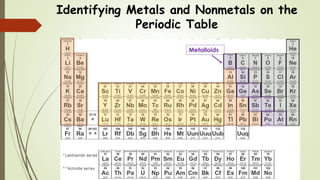
This document discusses the formation of ionic bonds between ions. Ionic bonds are electrostatic attractions between oppositely charged ions. Cations are positively charged ions formed when metals lose electrons. Anions are negatively charged ions formed when nonmetals gain electrons. Ionic bonds generally form between metallic cations and nonmetallic anions. The document provides examples of common cations and anions and identifies metals, nonmetals, and metalloids on the periodic table.