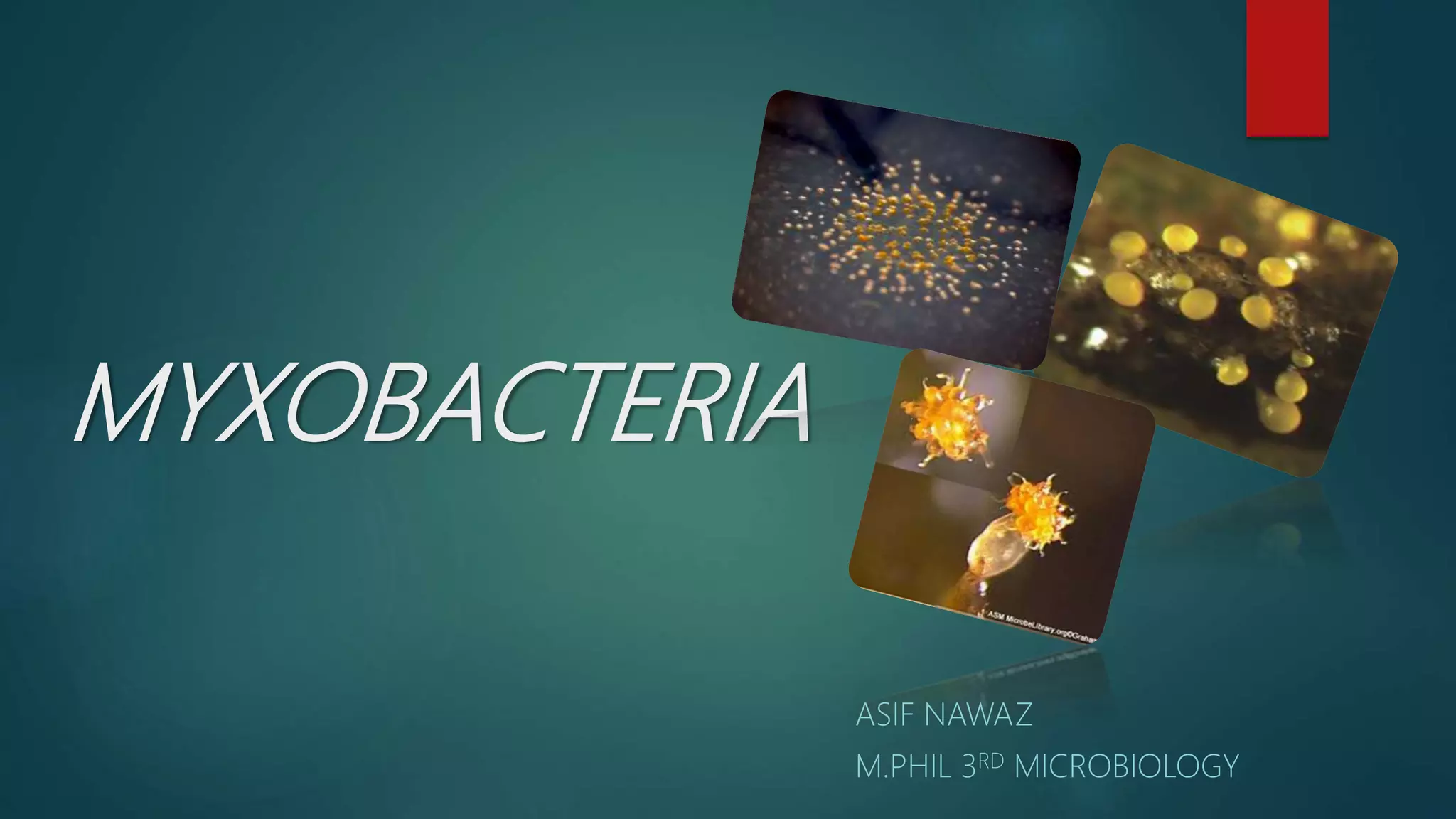
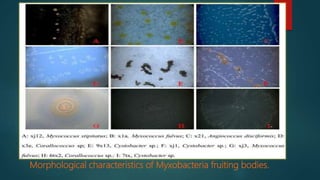
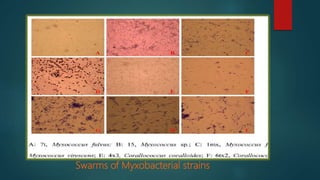
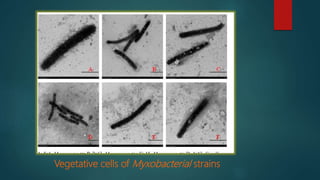
Myxobacteria, known as 'slime bacteria,' are soil-dwelling organisms with large genomes and unique life cycles, including the ability to form fruiting bodies and engage in predatory behavior. They can be isolated through various methods, such as baiting with dung and using E. coli, and are characterized morphologically to determine their taxonomy. Additionally, myxobacteria produce valuable secondary metabolites with antibacterial, antifungal, and anticancer properties, making them significant in various applications, including agriculture and medicine.