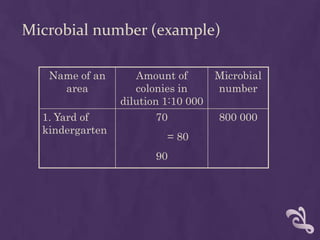
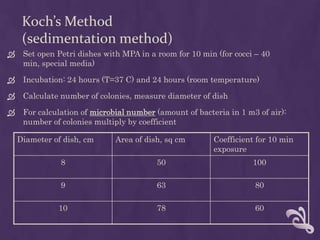
This document summarizes methods for analyzing the microflora of soil, water, and air. Soil samples are taken and diluted to determine the total microbial number and presence of indicator bacteria like E. coli. Water samples are filtered and cultured to determine totals and detect pathogens. Air samples use sedimentation or impaction to capture microbes and culture them to identify nosocomial pathogens. Proper sampling, transport, and dilution techniques are described to accurately assess microfloral conditions.