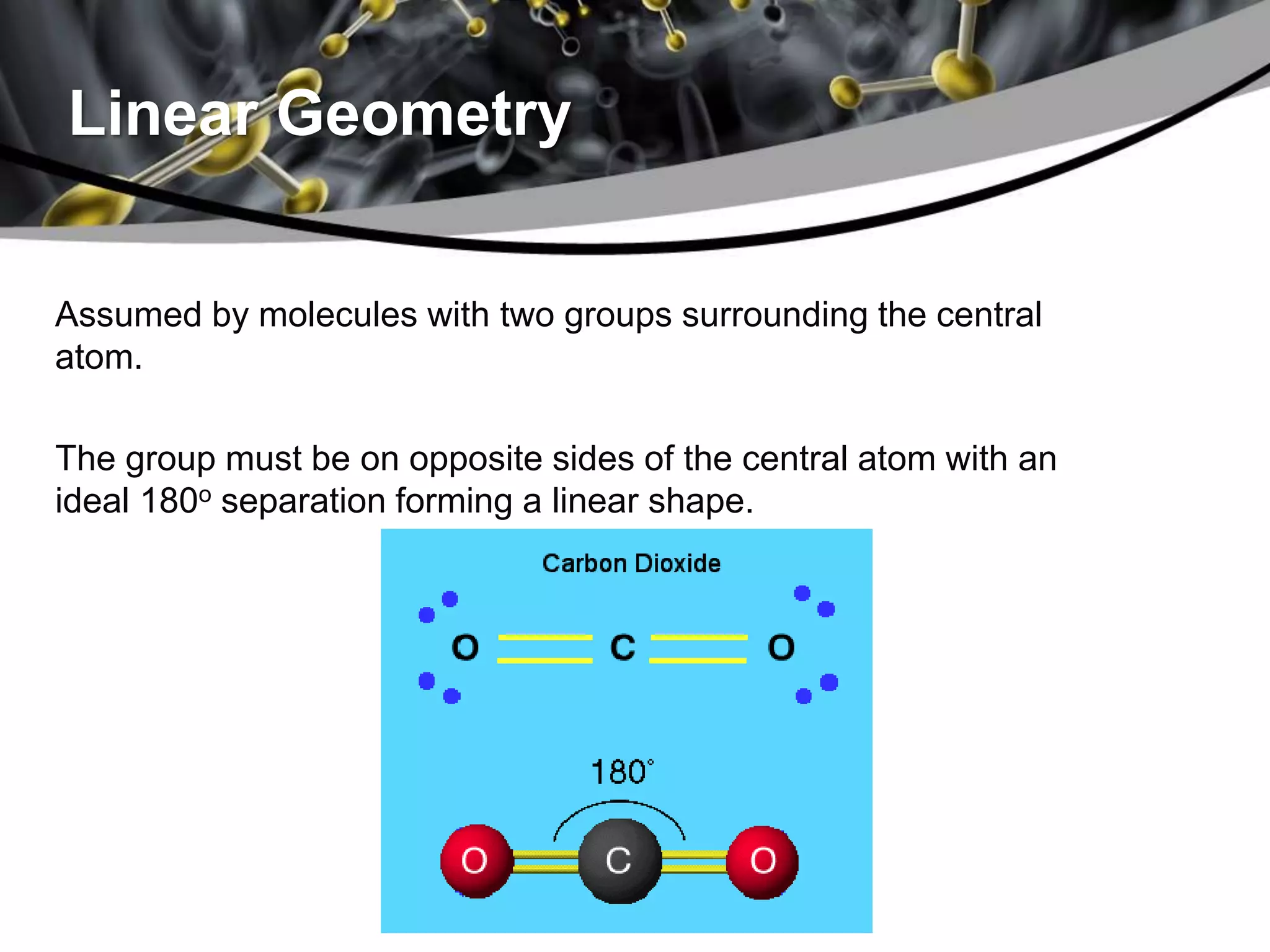
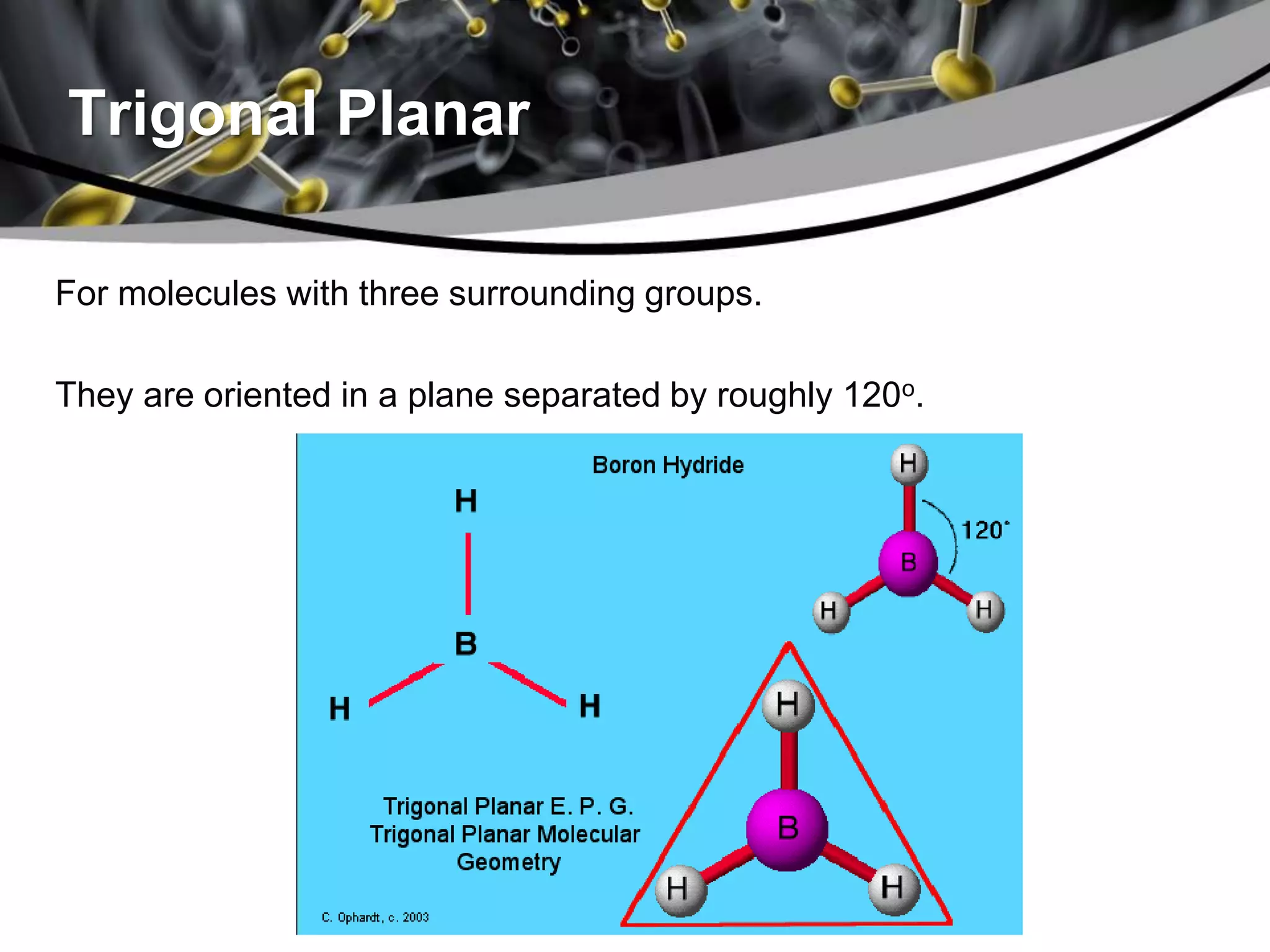
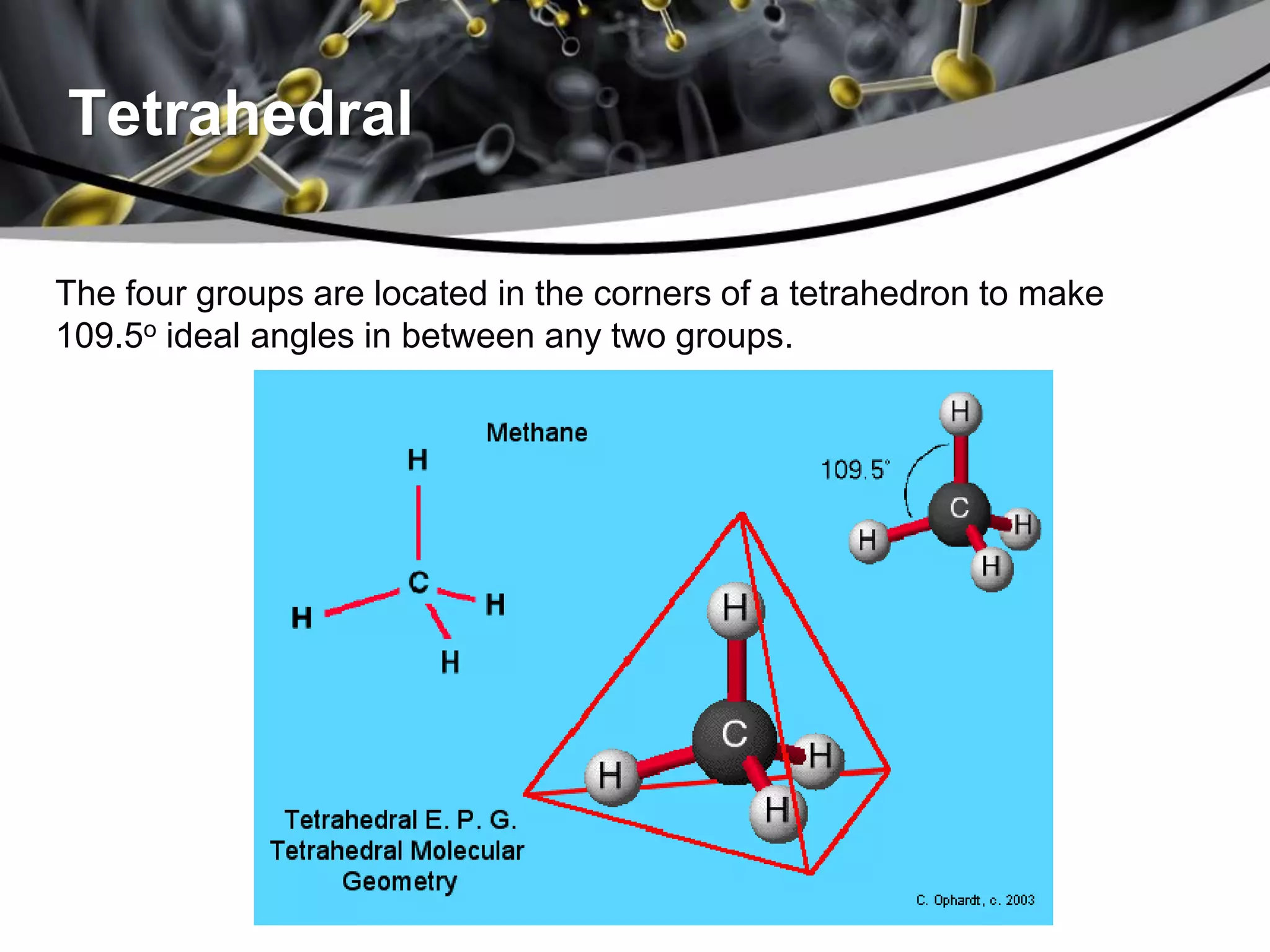
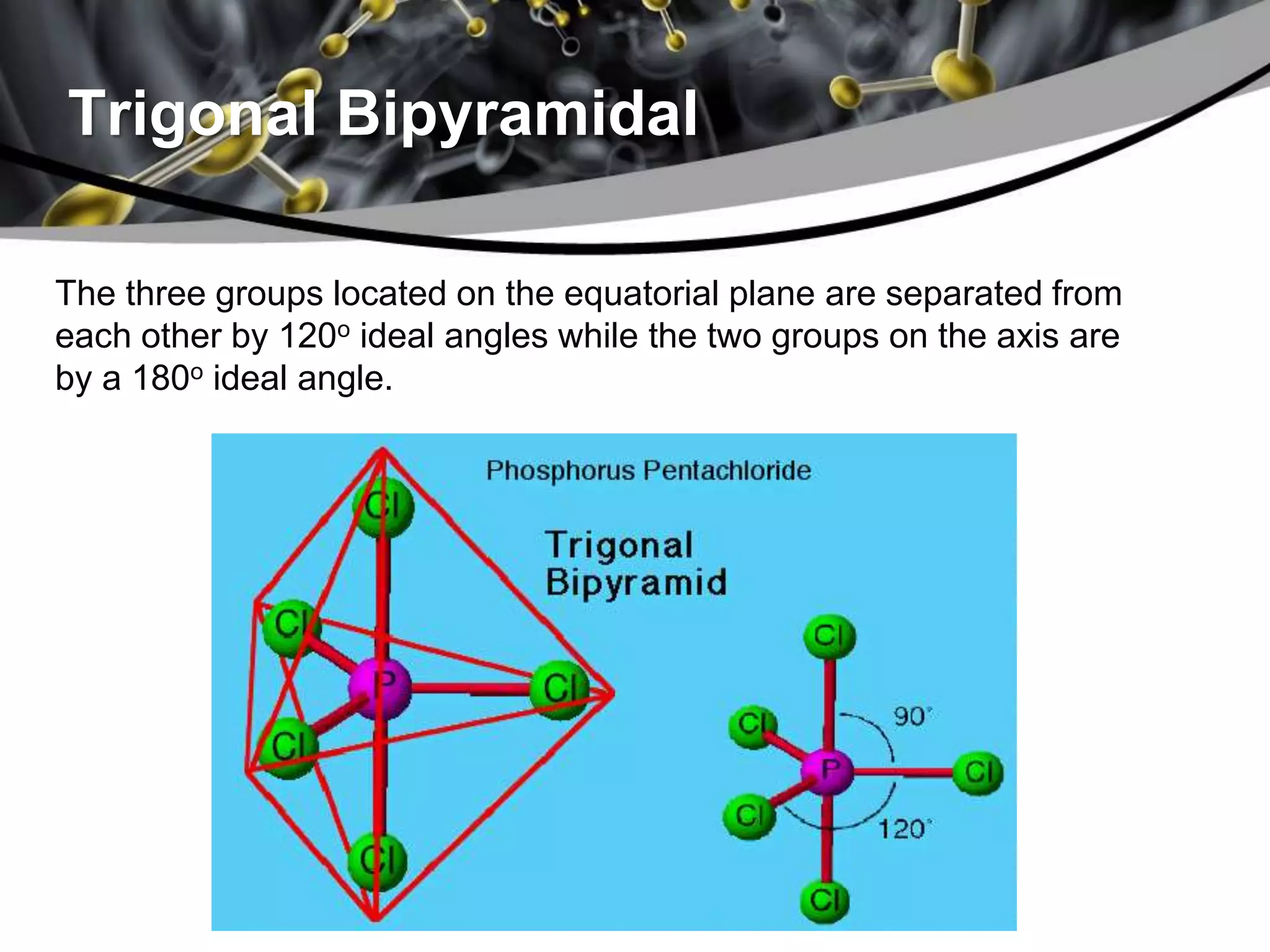
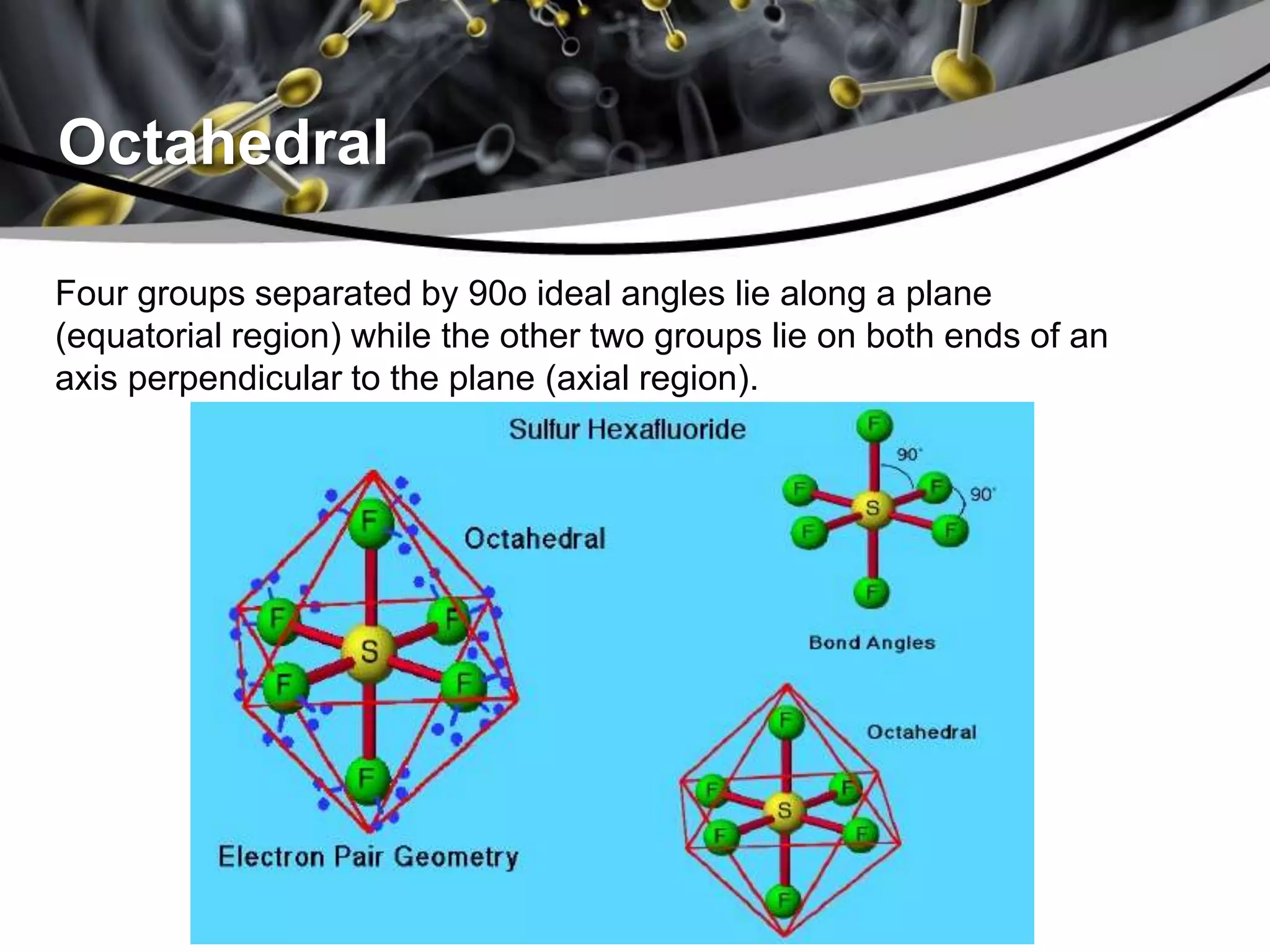
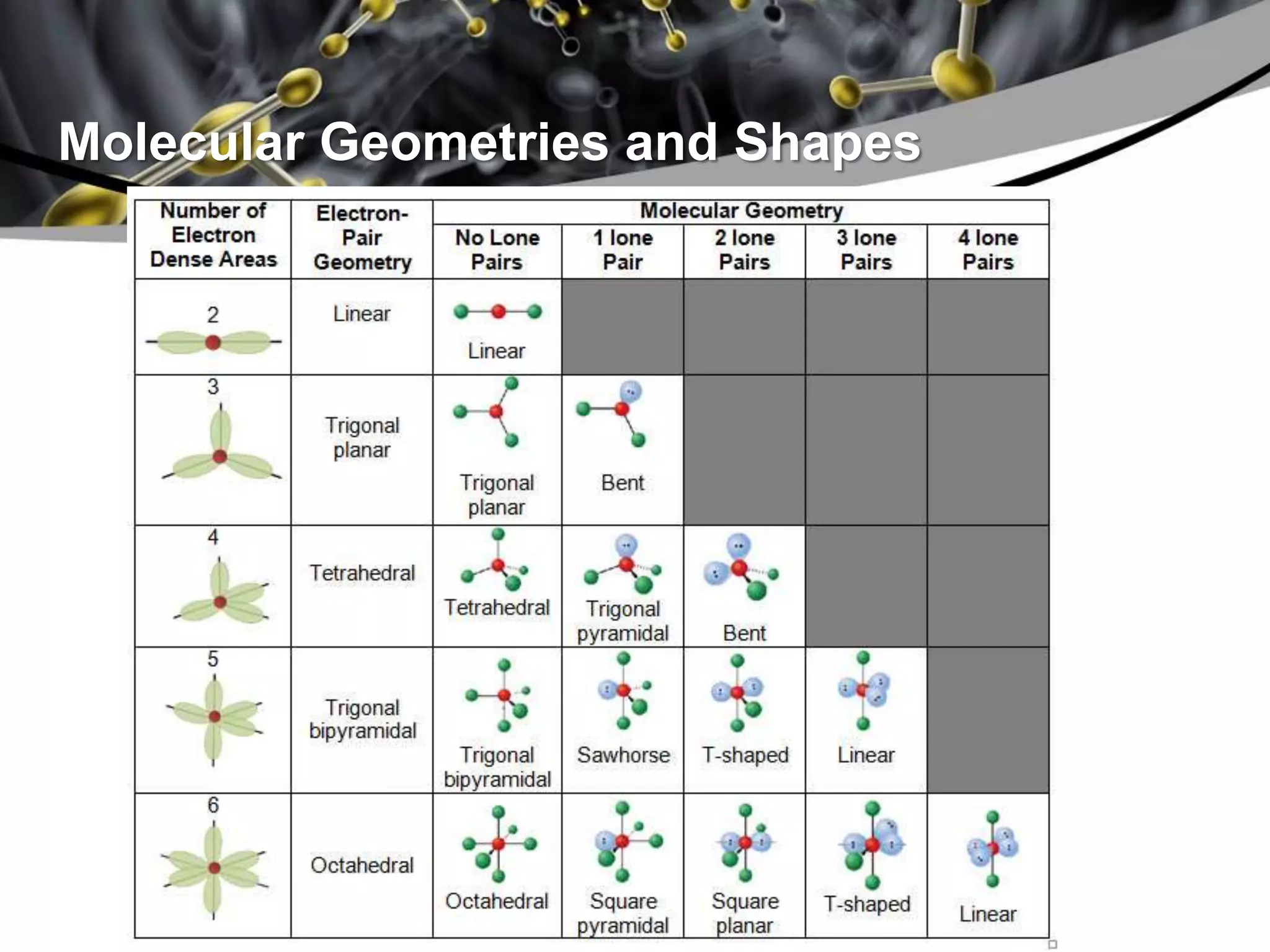
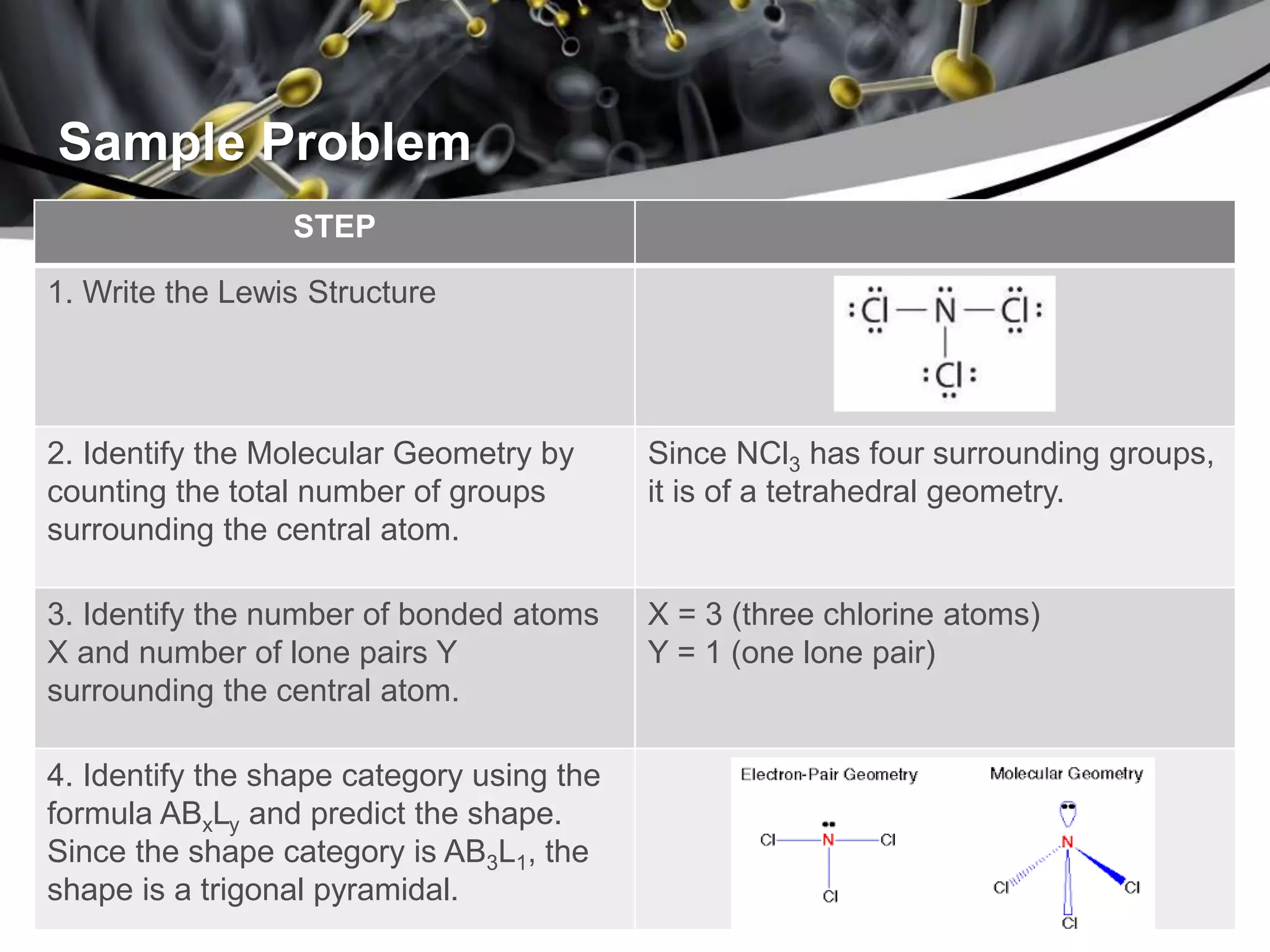
This document discusses molecular structures and geometry using Valence Shell Electron Pair Repulsion (VSEPR) Theory. It defines VSEPR Theory, describes common molecular geometries including linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral. It also distinguishes between molecular geometry defined by the total number of groups around a central atom, and molecular shape which considers just the bonded atoms. A sample problem demonstrates how to use VSEPR Theory to determine the geometry and shape of NCl3.