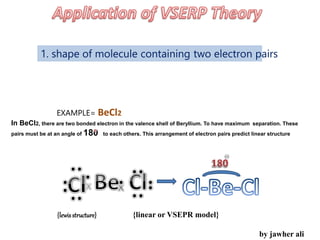
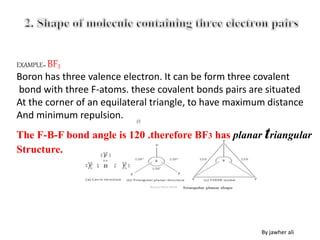
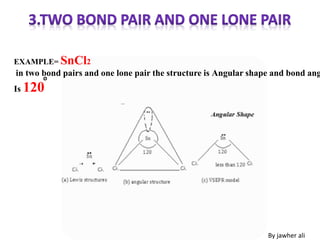
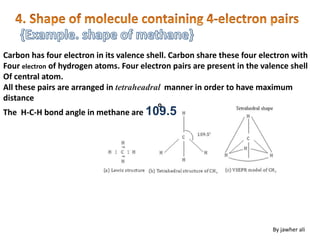
(1) VSEPR (Valence Shell Electron Pair Repulsion) theory predicts molecular geometry based on the number of electron pairs around a central atom and their repulsions.
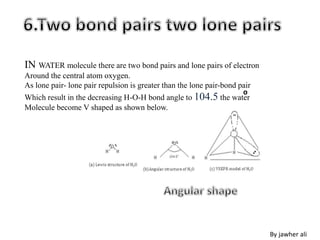
(2) Electron pairs arrange themselves as far apart from each other as possible to minimize repulsion. Lone pairs occupy more space than bonding pairs due to repulsion from one nucleus.
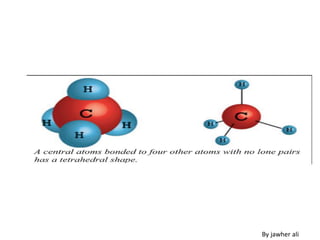
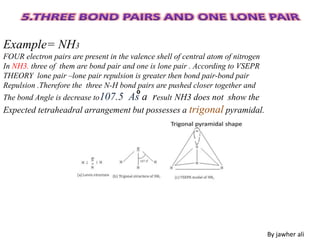
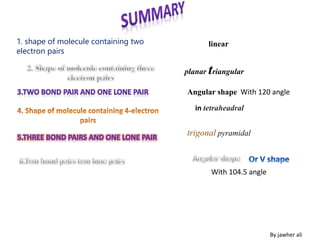
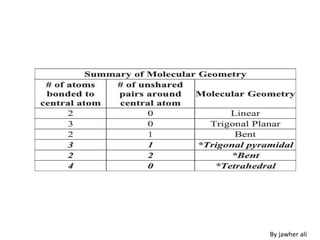
(3) Molecular geometry depends on the number of electron pairs. Examples include linear for two pairs, trigonal planar for three pairs, and tetrahedral for four pairs. The presence of lone pairs can distort the geometry from the ideal shape.