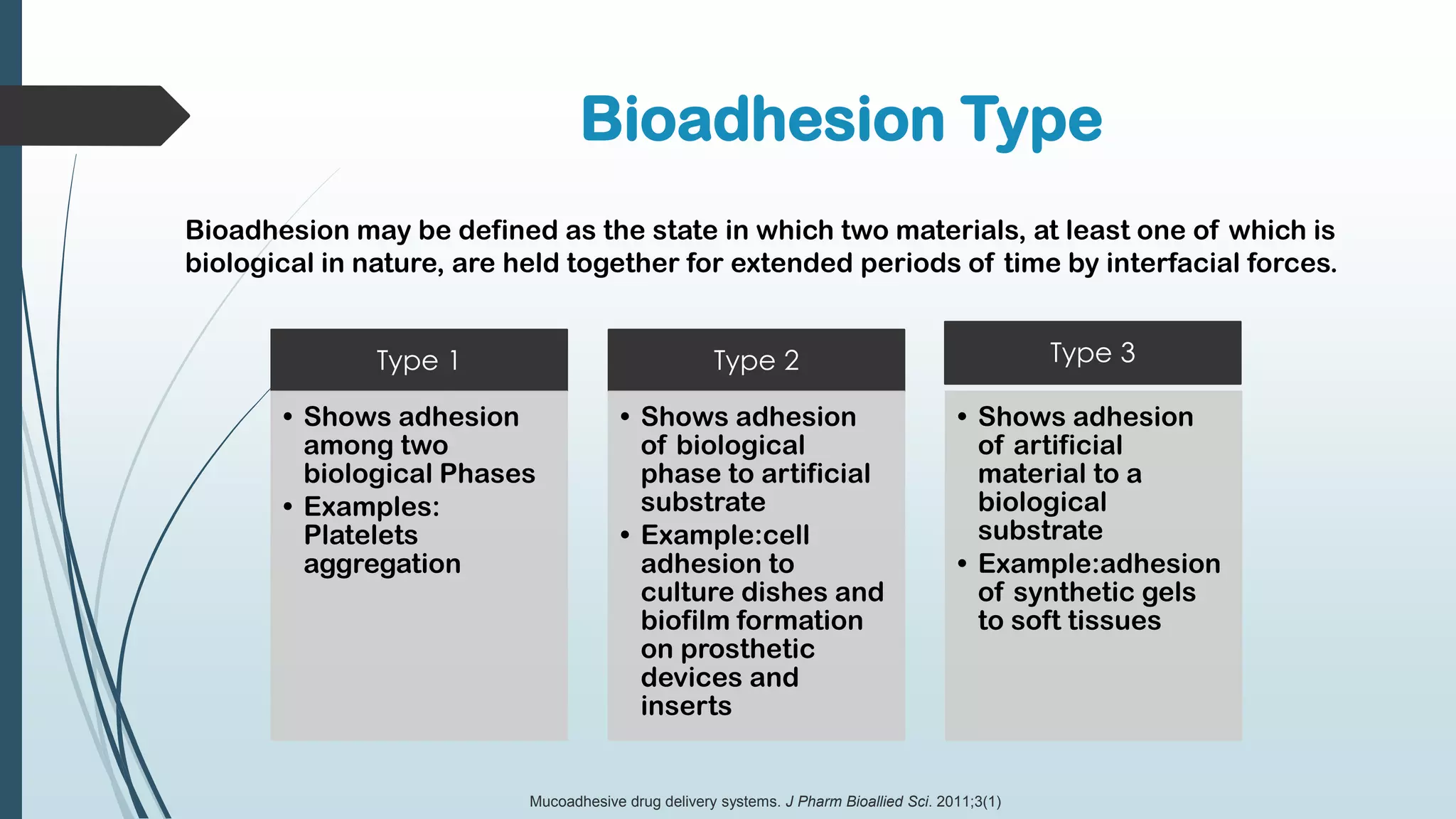
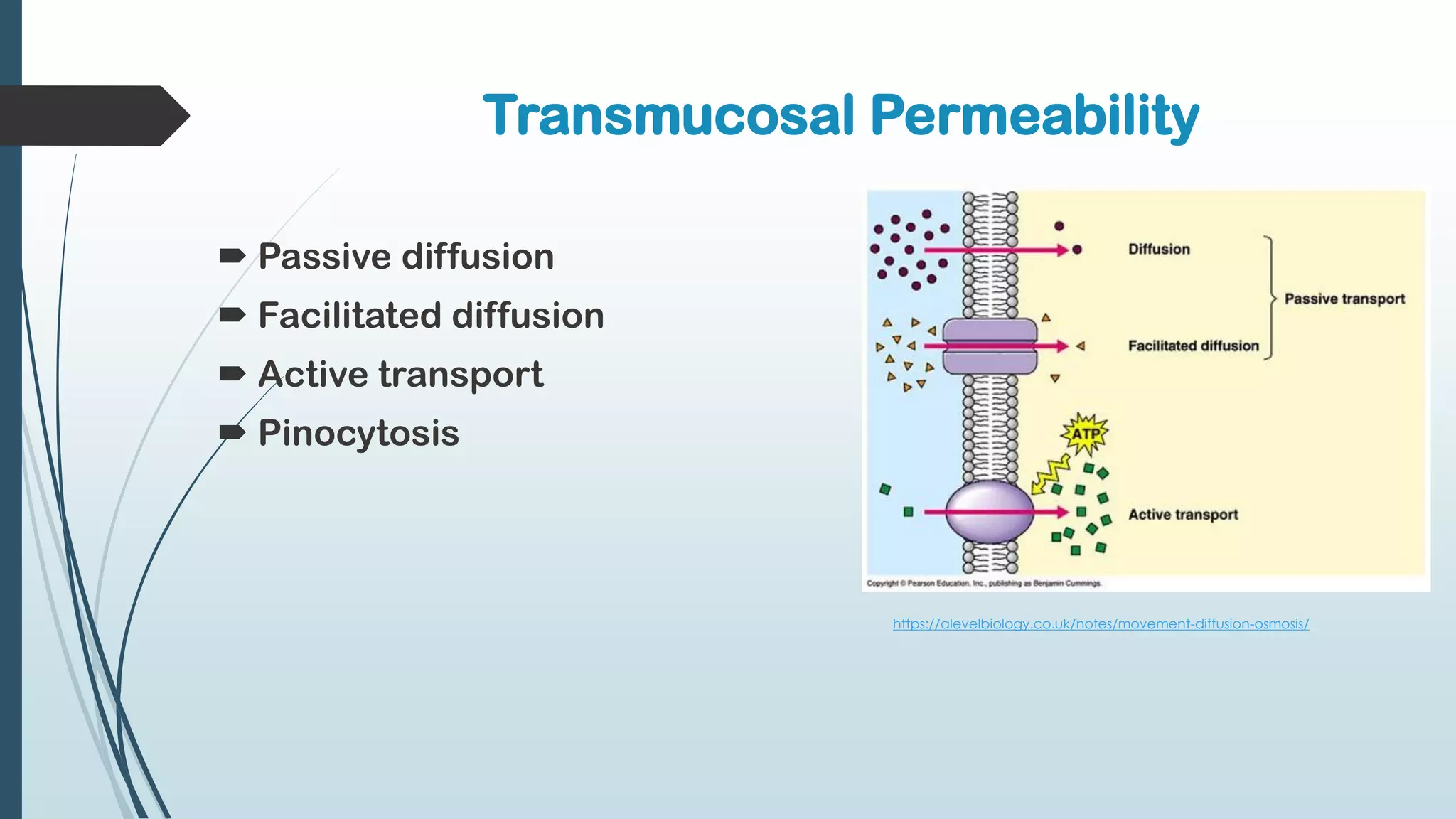
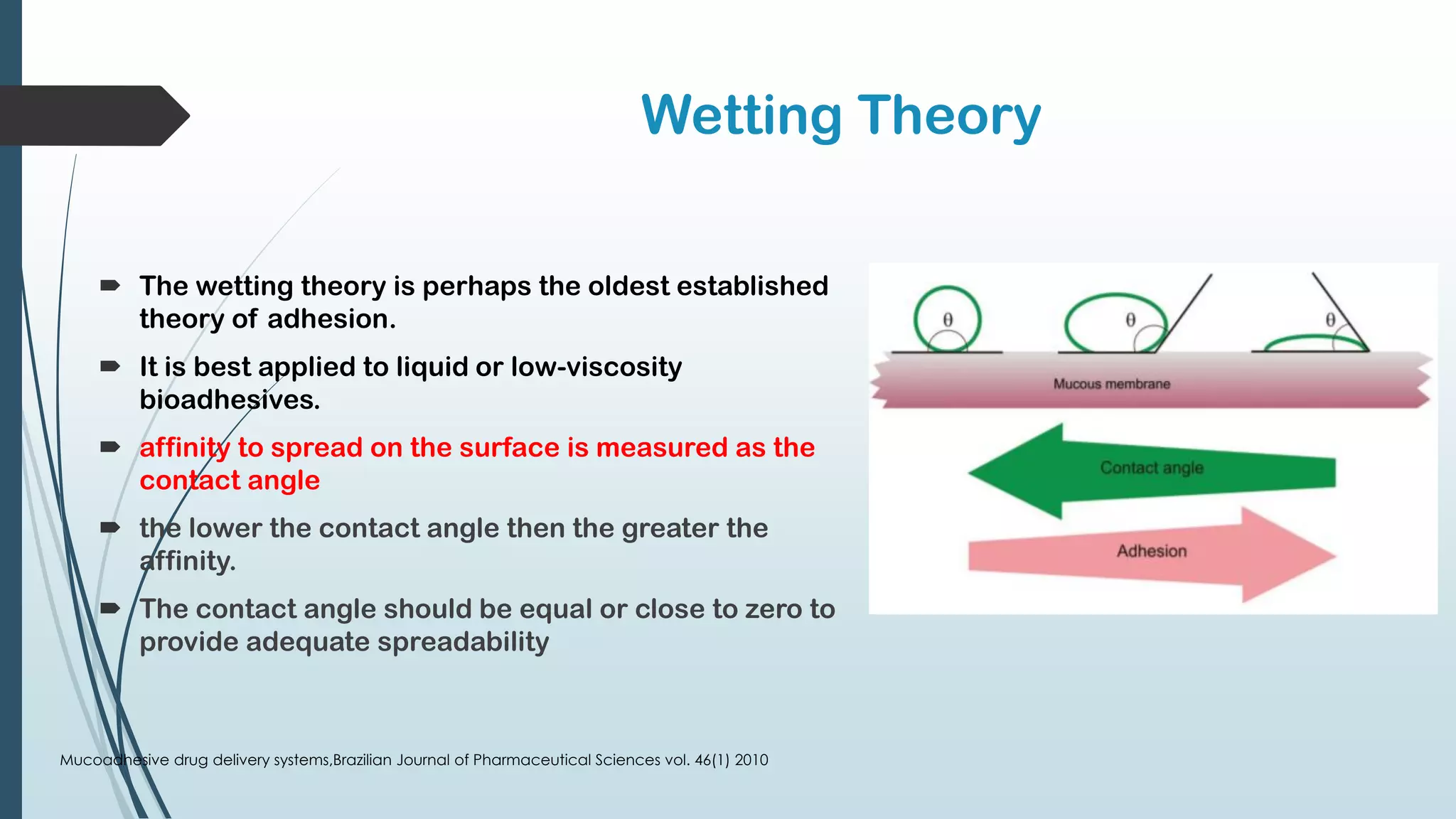
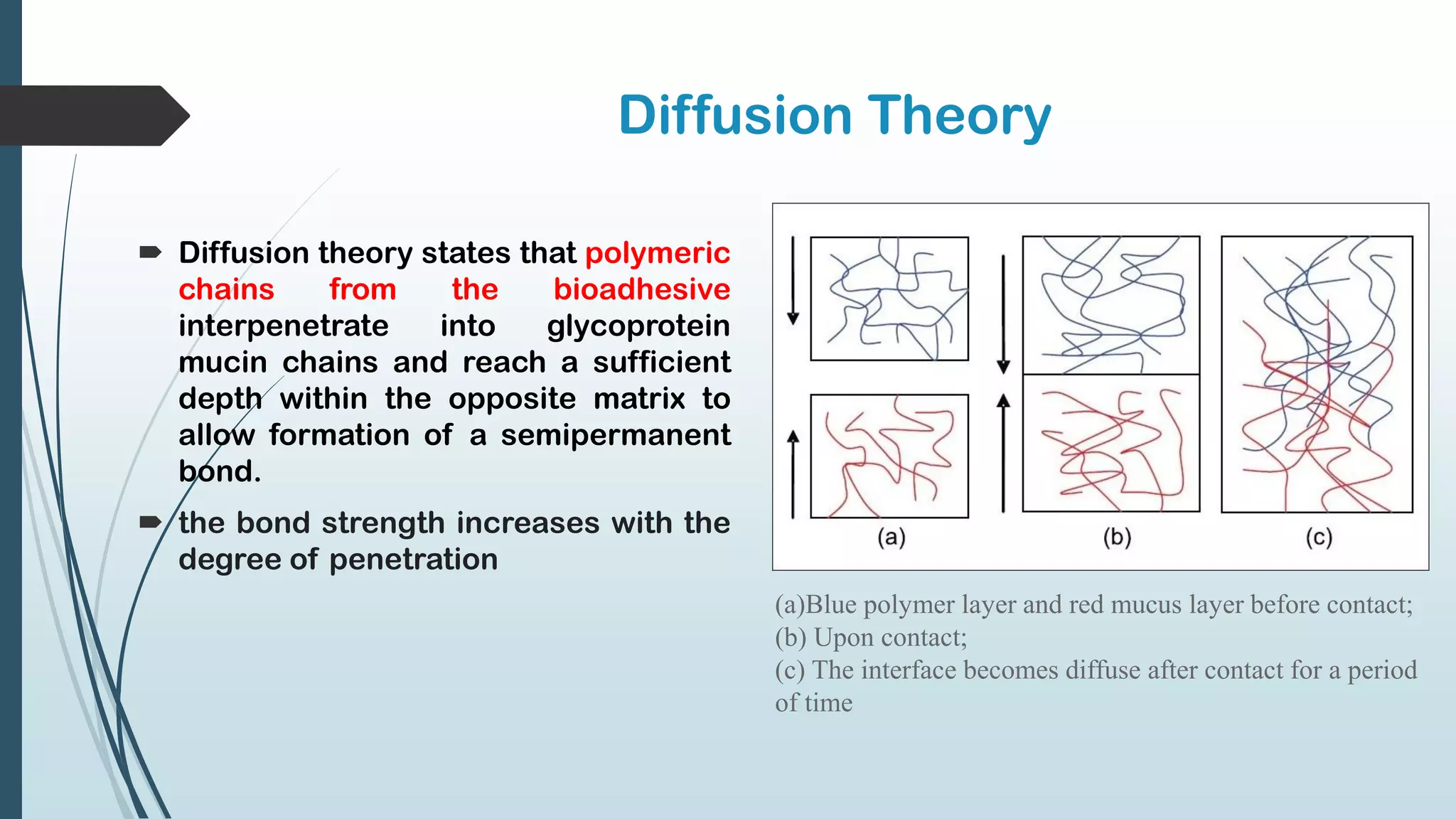
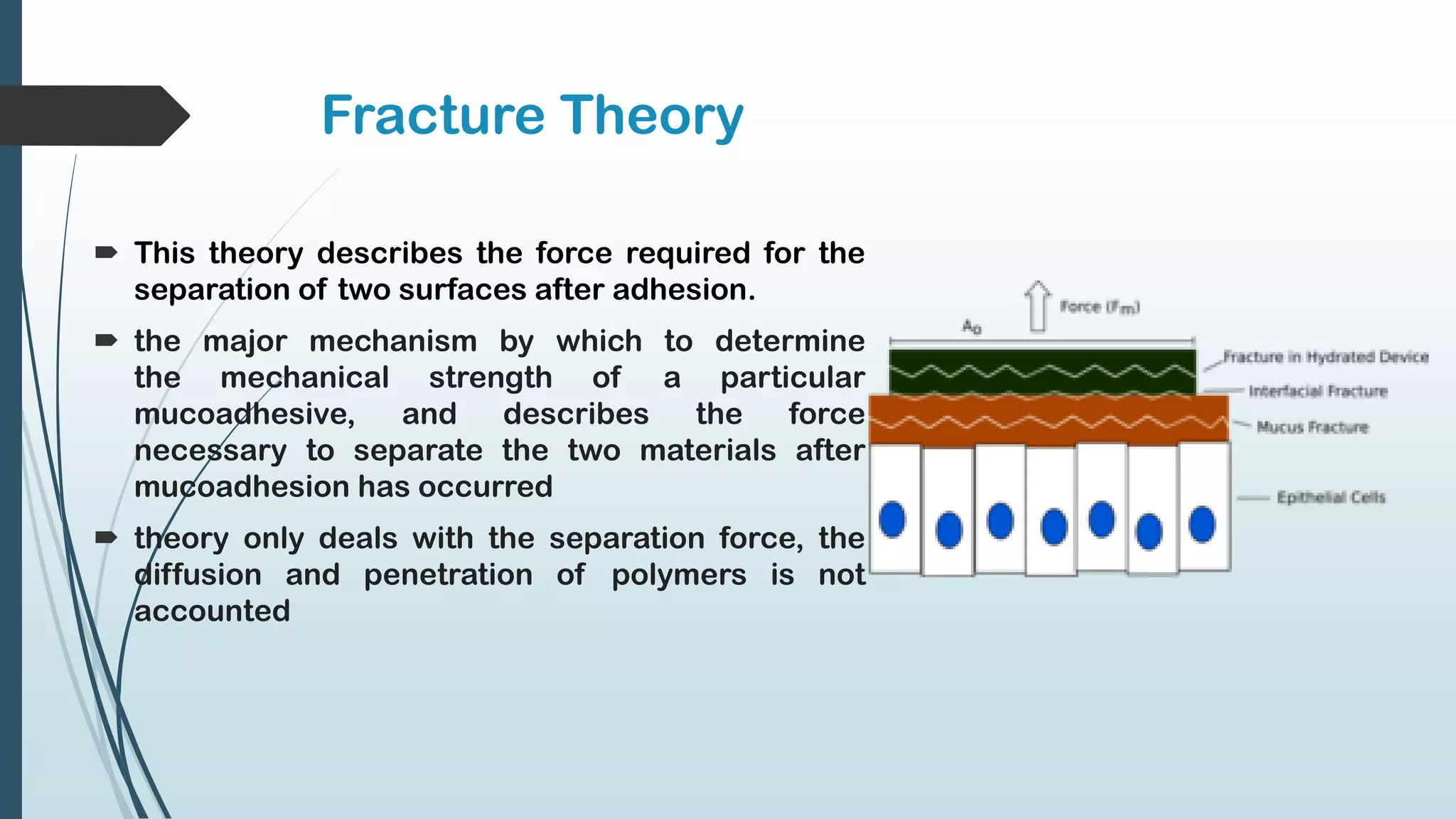
This document discusses mucosal drug delivery systems. It defines mucosal drug delivery as systems that interact with mucosal layers to increase the residence time of formulations for better drug absorption. The principles of bioadhesion and mucoadhesion are described, along with the advantages of these systems like improved bioavailability and reduced dosing frequency. Various mucosal routes of administration like buccal, sublingual, ocular and nasal are mentioned. Key factors that influence transmucosal permeability like drug properties, environmental conditions and permeation enhancers are summarized. Common bioadhesive polymers used in these systems like chitosan, polyacrylic acid and cellulose derivatives are also outlined.