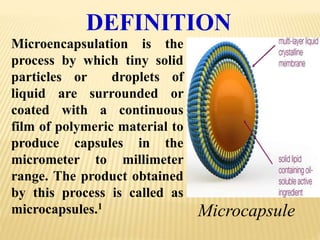
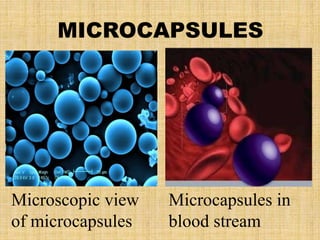
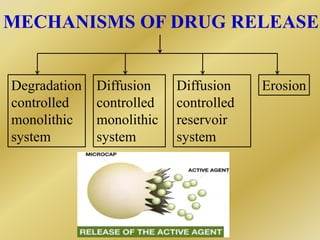
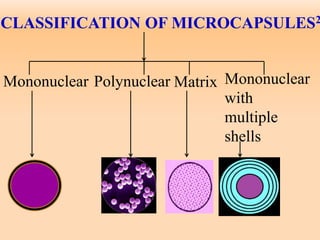
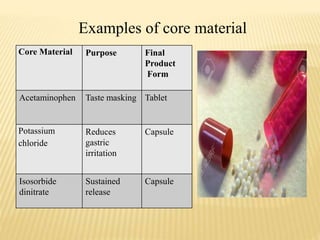
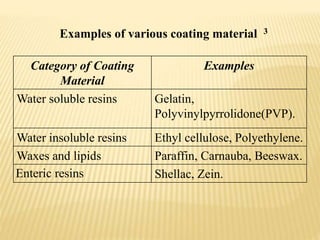
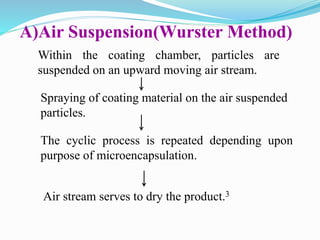
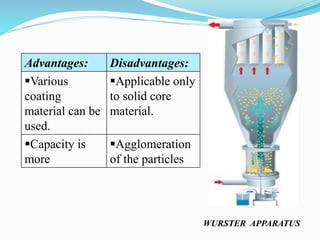
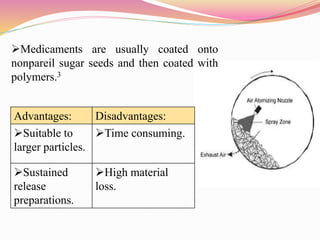
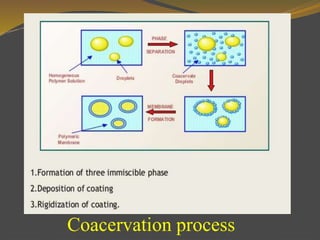
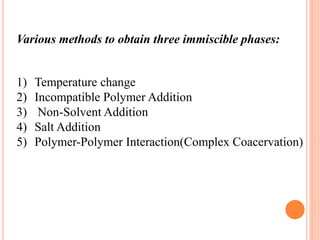
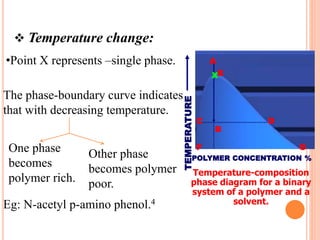
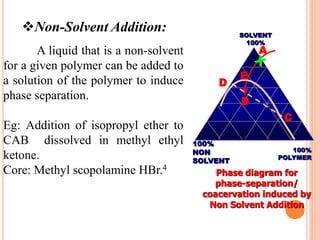
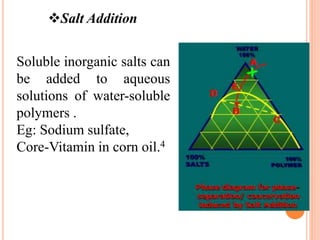
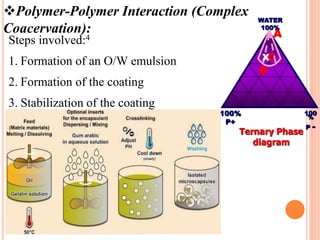
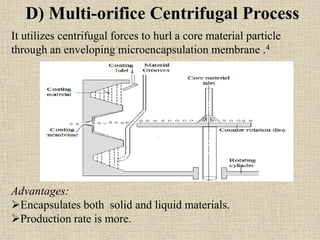
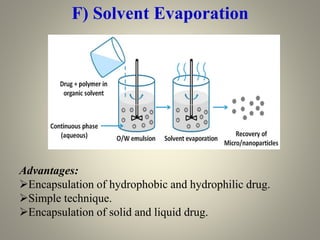
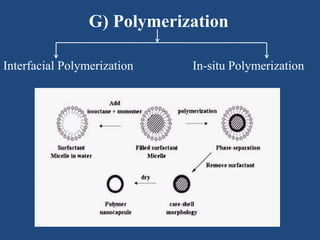
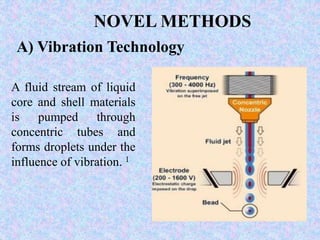
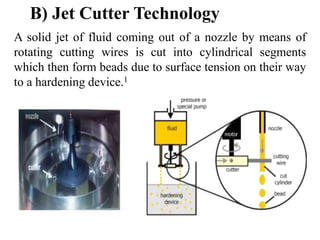
Microencapsulation is a process where tiny particles or droplets of a core material are surrounded by a coating to form capsules in the micrometer to millimeter range called microcapsules. Various techniques are used to produce microcapsules including air suspension, pan coating, coacervation, solvent evaporation, and polymerization. Microencapsulation offers advantages like taste masking, sustained release, and protection of materials. Microcapsules find applications in pharmaceuticals for controlled drug delivery and replacement of non-oral therapeutics. Some commercial products that use microencapsulation technology include Lupin Cefadroxil, ZORprin CR, and Glipizide SR.