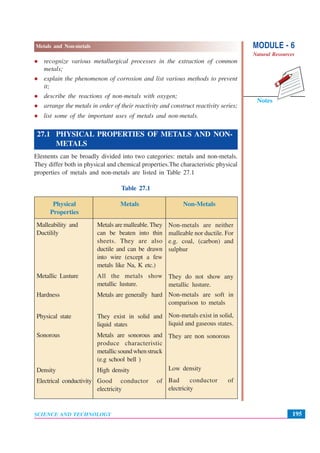
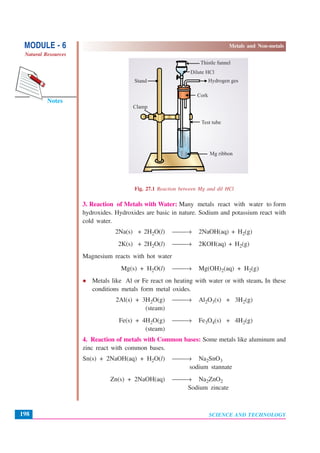
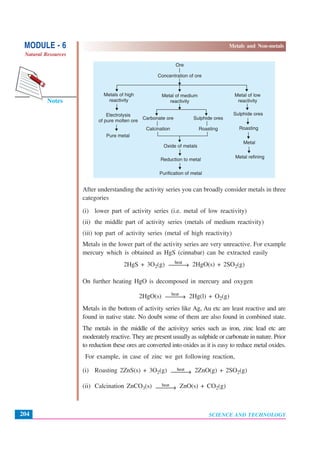
This document discusses metals and non-metals. It begins by explaining how metals and non-metals surround us in daily life through various tools and containers. It then discusses the physical properties that differentiate metals from non-metals, such as malleability, lustre, hardness, state of matter, conductivity. It also explains some chemical properties such as how metals react with oxygen, acids, water and bases. Common reactions include the formation of metal oxides, hydrogen gas, and hydroxides. Corrosion is discussed as the oxidation of metals over time. The document aims to help students understand the differences between metals and non-metals.