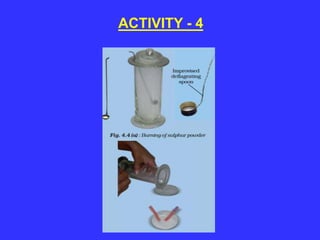
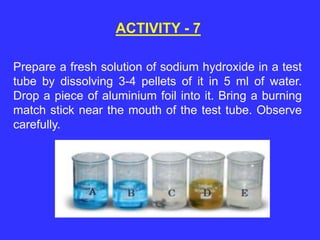
The document discusses the properties and characteristics of metals and non-metals, focusing on their physical and chemical attributes such as malleability, ductility, conductivity, and reactivity. It includes various activities for students to observe these properties firsthand, emphasizing the distinctions between metals and non-metals, and providing examples and applications of each. Additionally, it introduces the concept of elements, highlighting that many substances are a combination of fewer than 92 naturally occurring elements, classified as metals or non-metals.