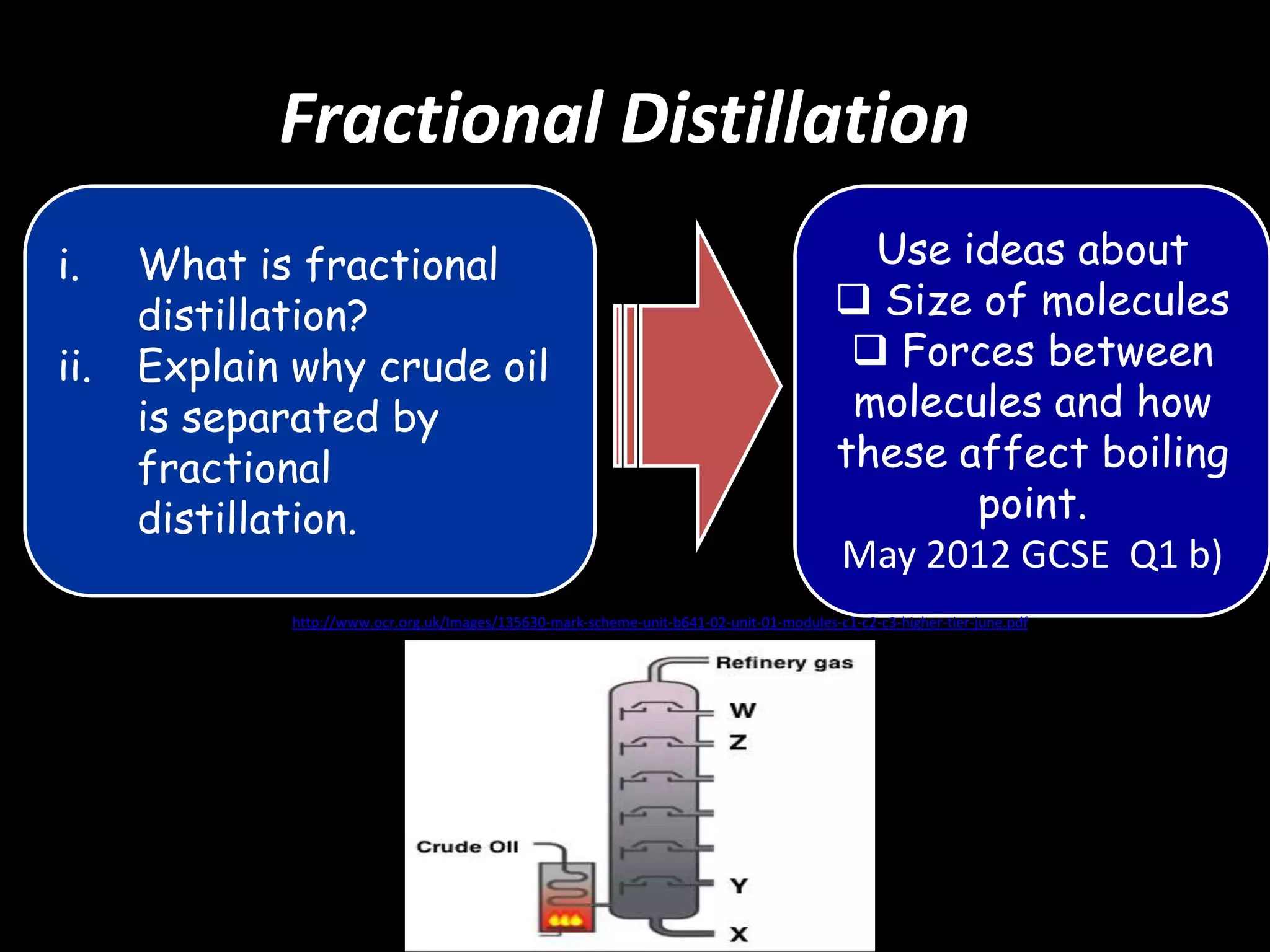
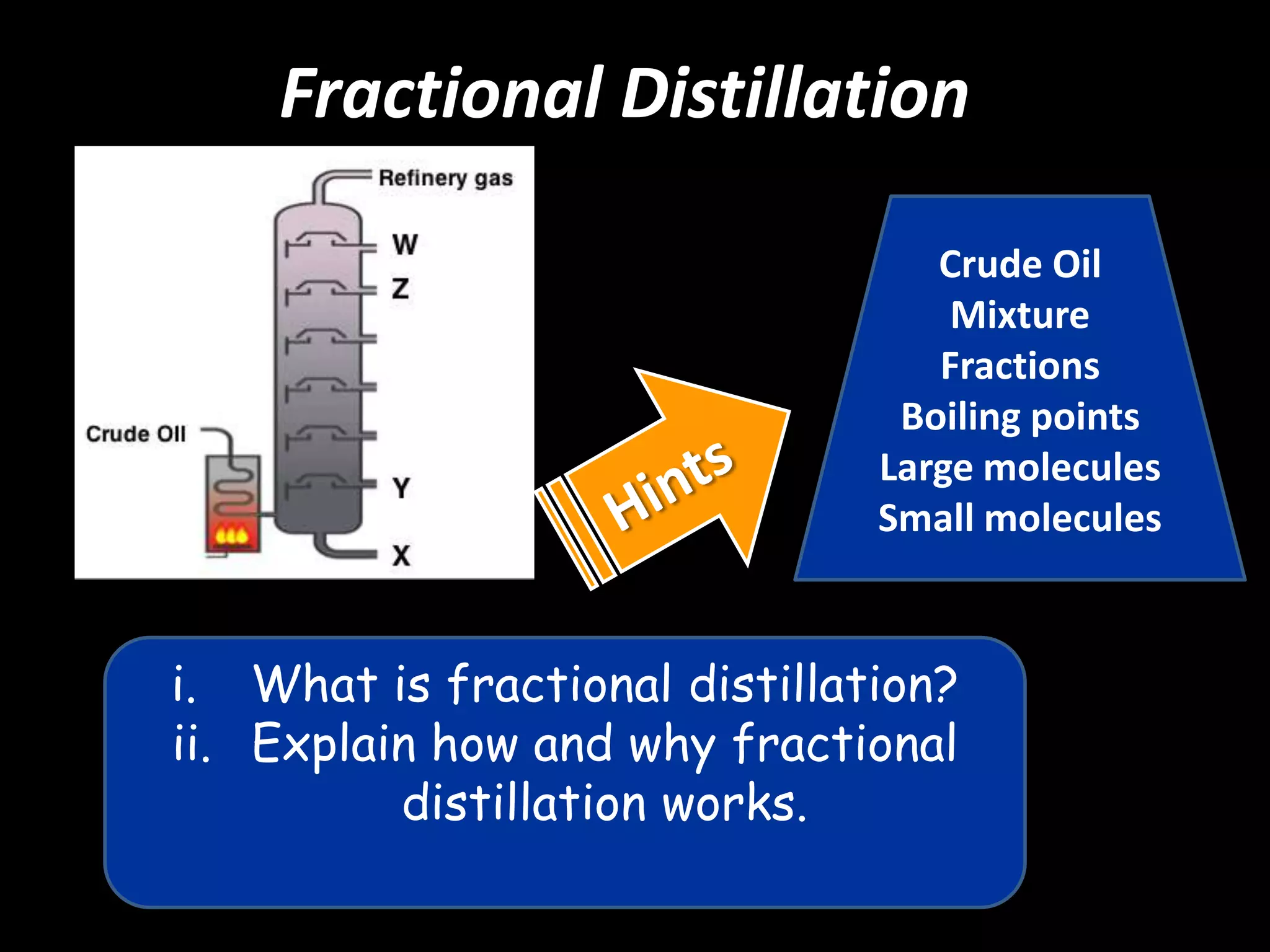
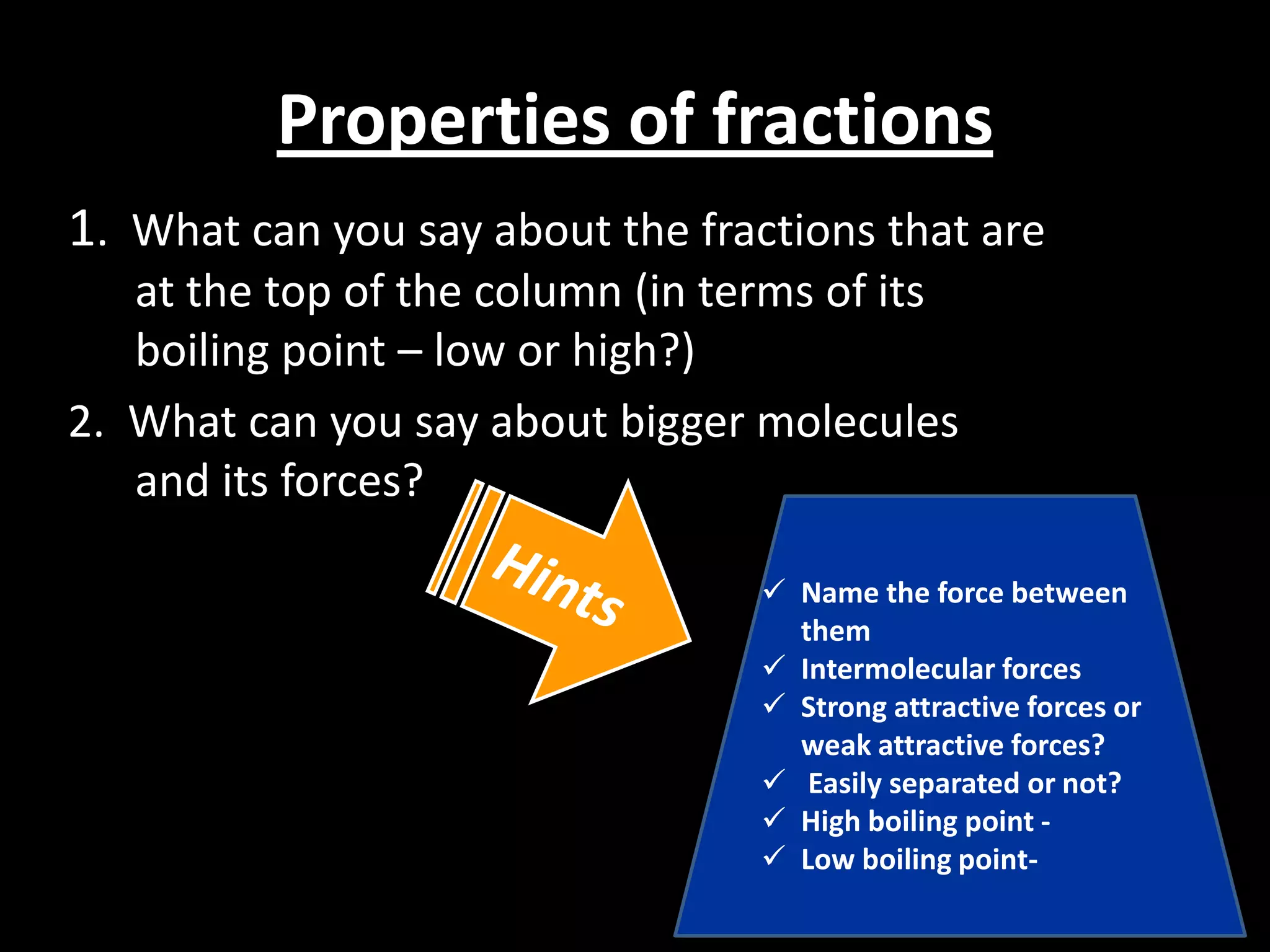
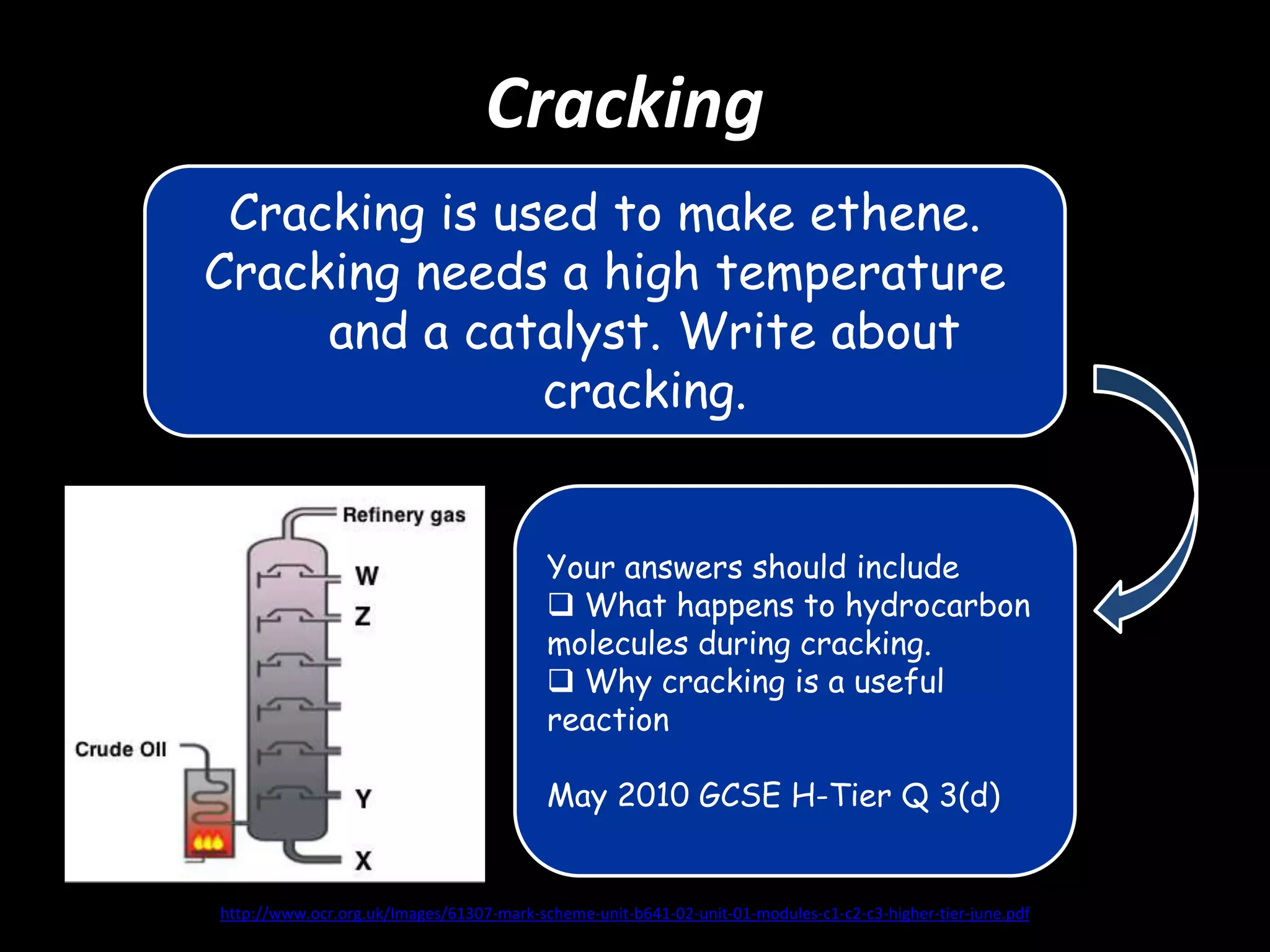
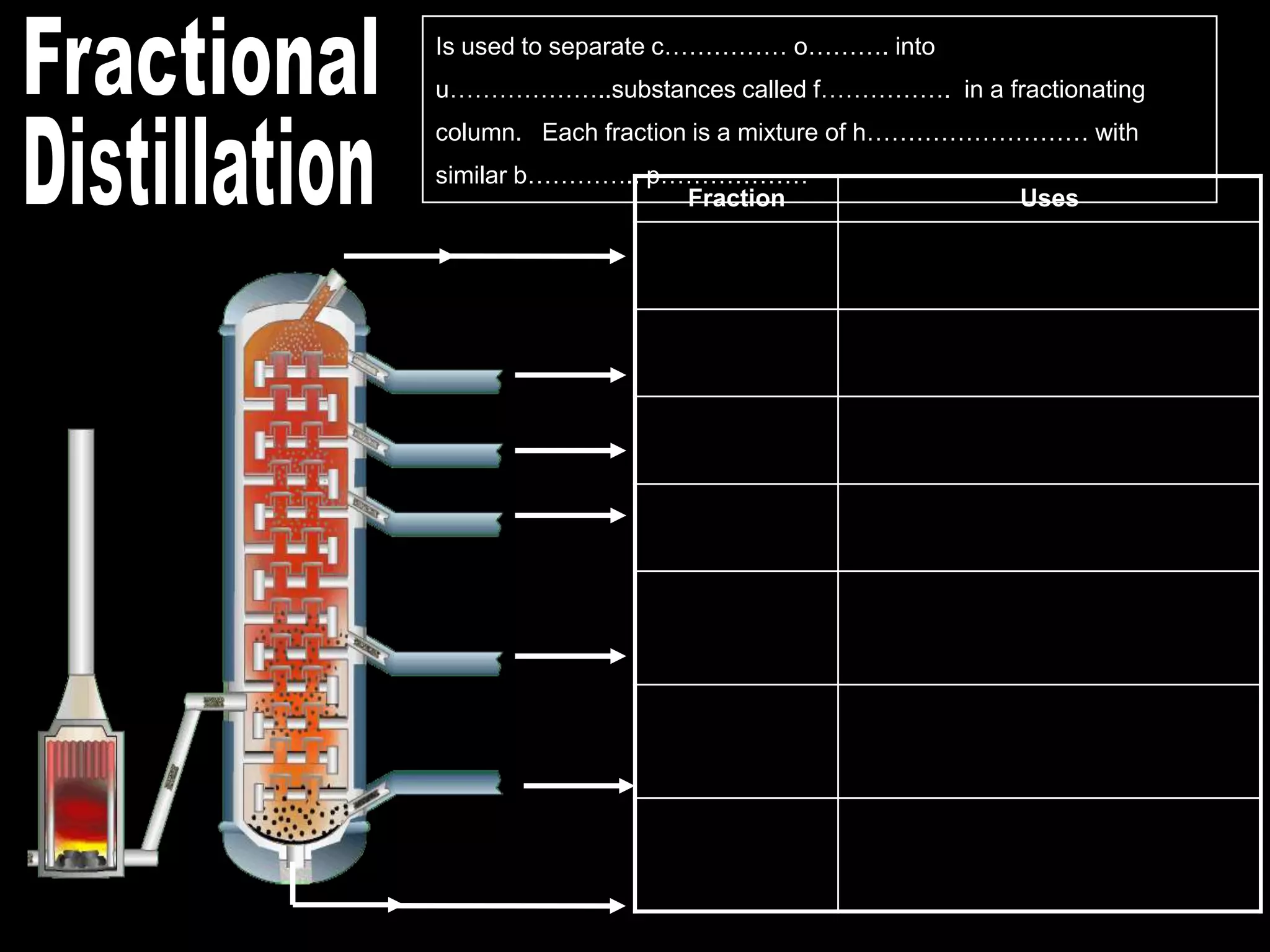
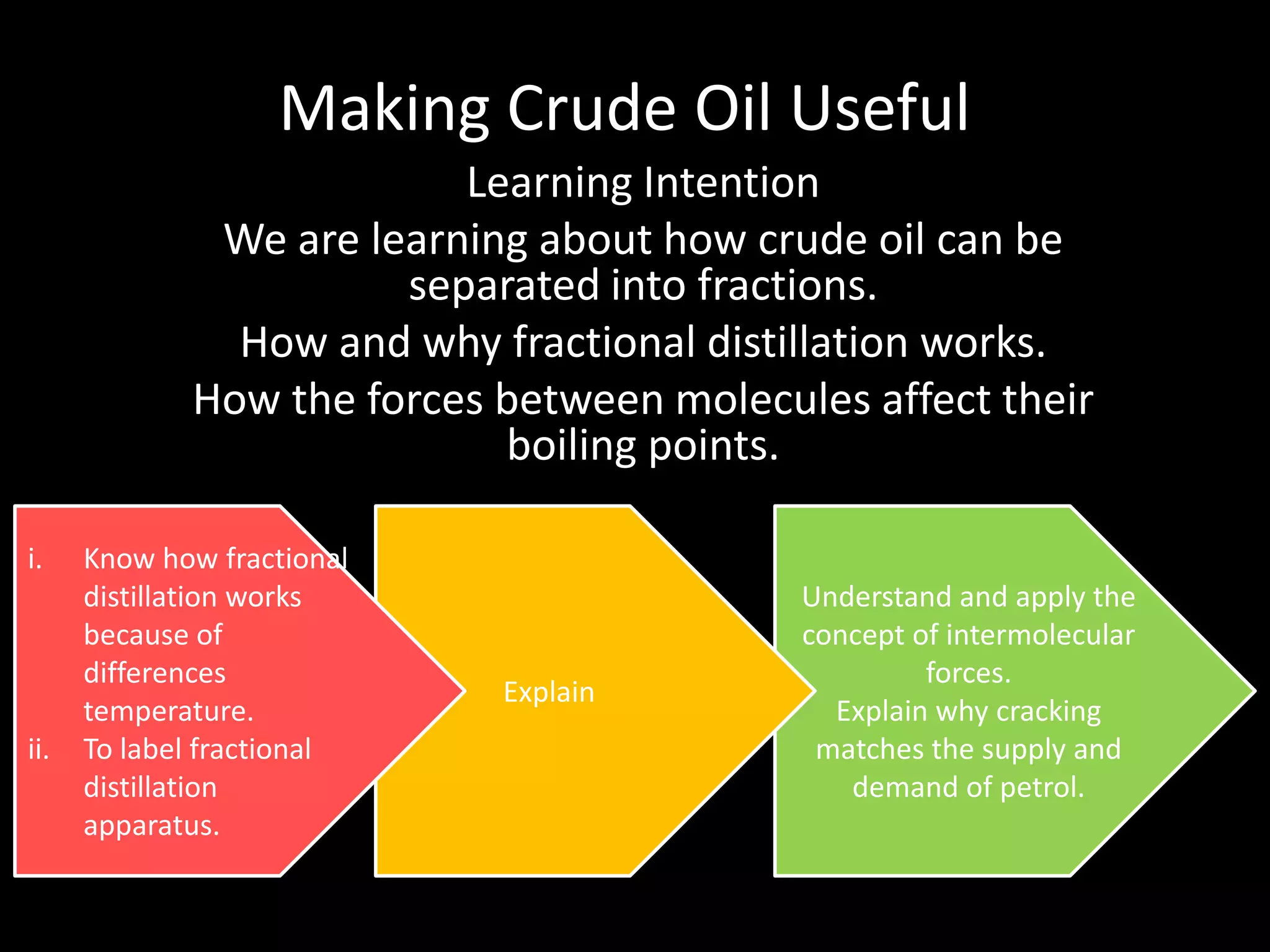
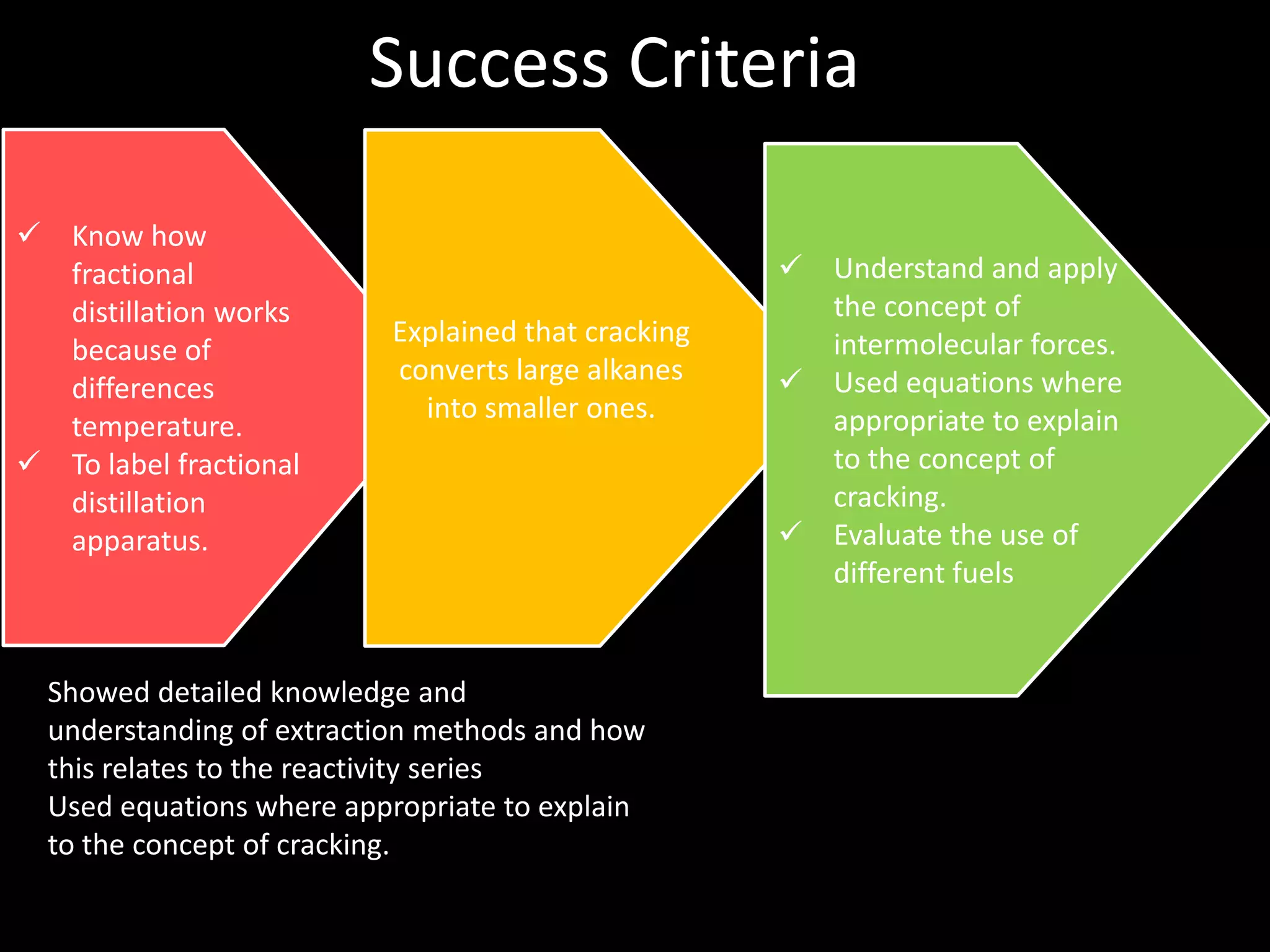
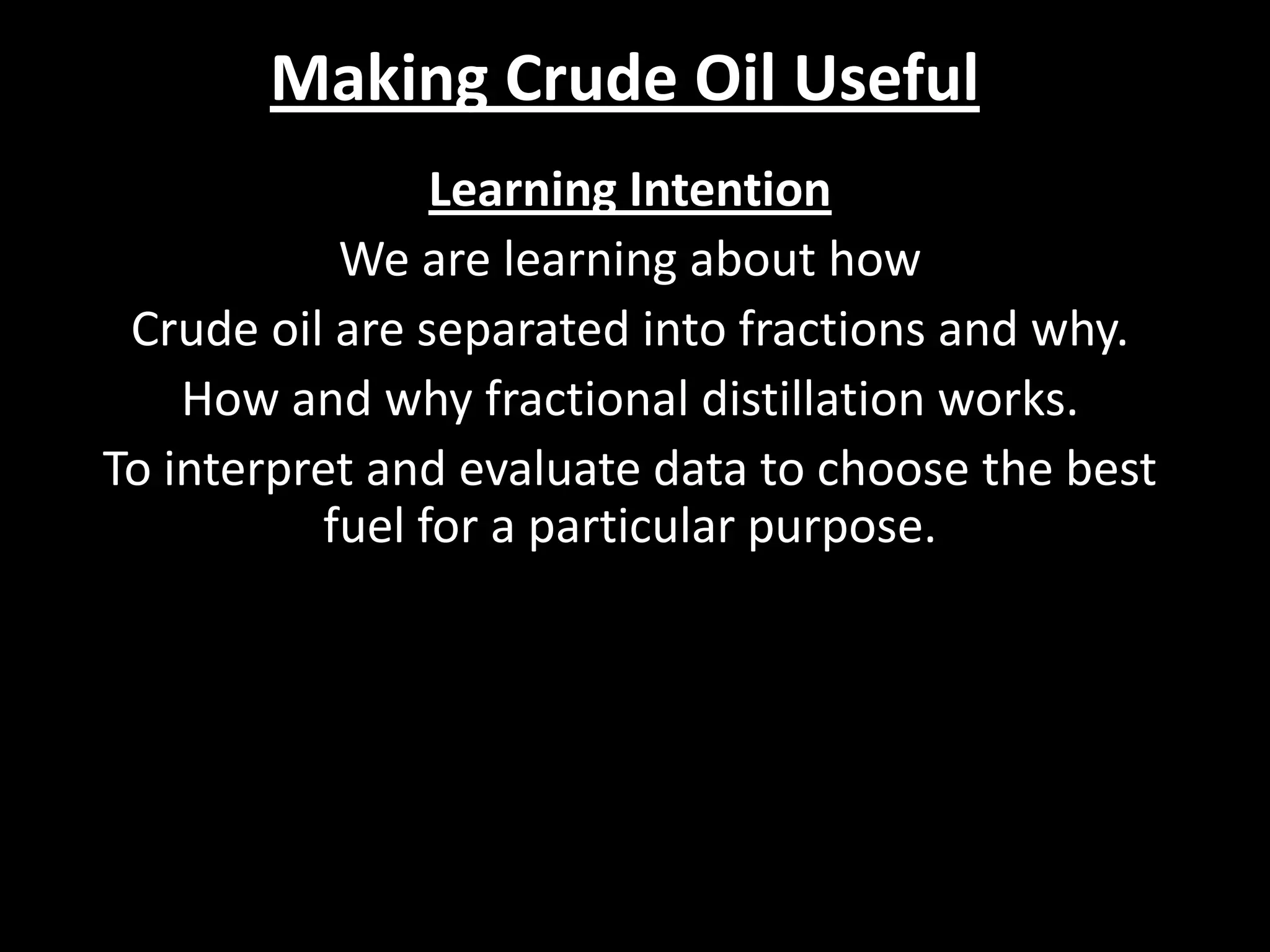
Crude oil is separated into fractions using fractional distillation. Fractional distillation works by using a fractionating column to separate different hydrocarbon molecules based on their varying boiling points, which are determined by the intermolecular forces between the molecules. Larger hydrocarbon molecules have stronger intermolecular forces and higher boiling points, so they remain lower in the fractionating column, while smaller molecules have weaker intermolecular forces and lower boiling points, so they travel higher in the column. Cracking breaks down larger hydrocarbon molecules into smaller, more useful molecules to meet demand for fuels since crude oil contains too many large molecules.