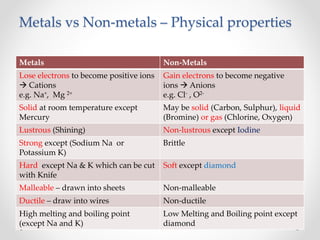
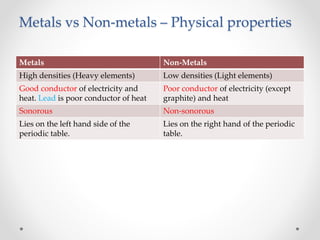
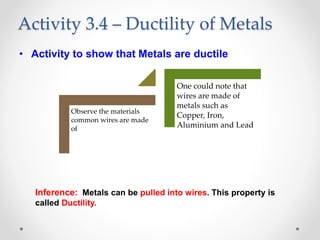
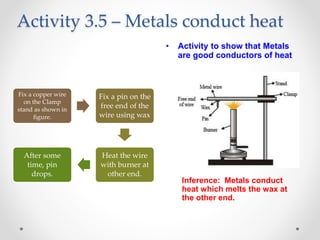
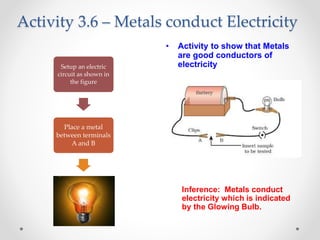
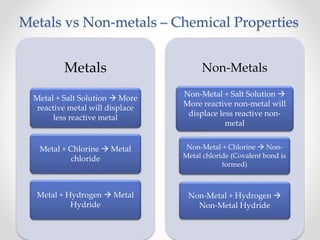
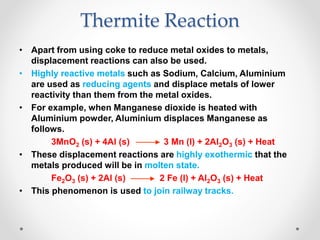
The document discusses the properties of metals and non-metals. It describes how metals are lustrous, malleable, ductile, and good conductors of heat and electricity, while non-metals lack these properties. Experiments are presented to demonstrate that metals are lustrous, hard except for a few, malleable by hammering into thin sheets, and ductile by pulling into wires. Other experiments show that metals conduct heat by melting wax and conduct electricity by lighting a bulb. The document contrasts how metals and non-metals react with oxygen, water, acids, and how metals react in salt solutions in displacement reactions.