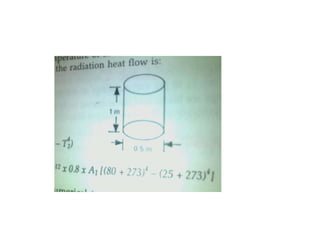
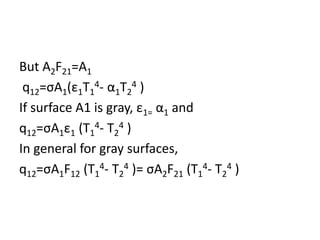1. Radiation can be described using both wave and particle theories, with photons traveling at the speed of light and having energy levels related to their frequency.
2. Thermal radiation emitted from surfaces is within the wavelength range of 10-7 to 10-4 m. The human eye can detect wavelengths from 3.8x10-7 to 7.6x10-7 m, known as visible radiation.
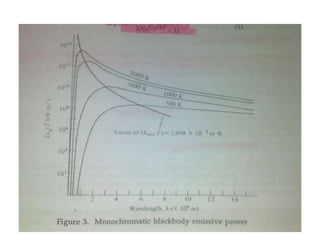
3. A blackbody is an idealized radiating surface that absorbs all radiation falling on it and reaches the maximum possible emissive power at each wavelength for a given temperature.