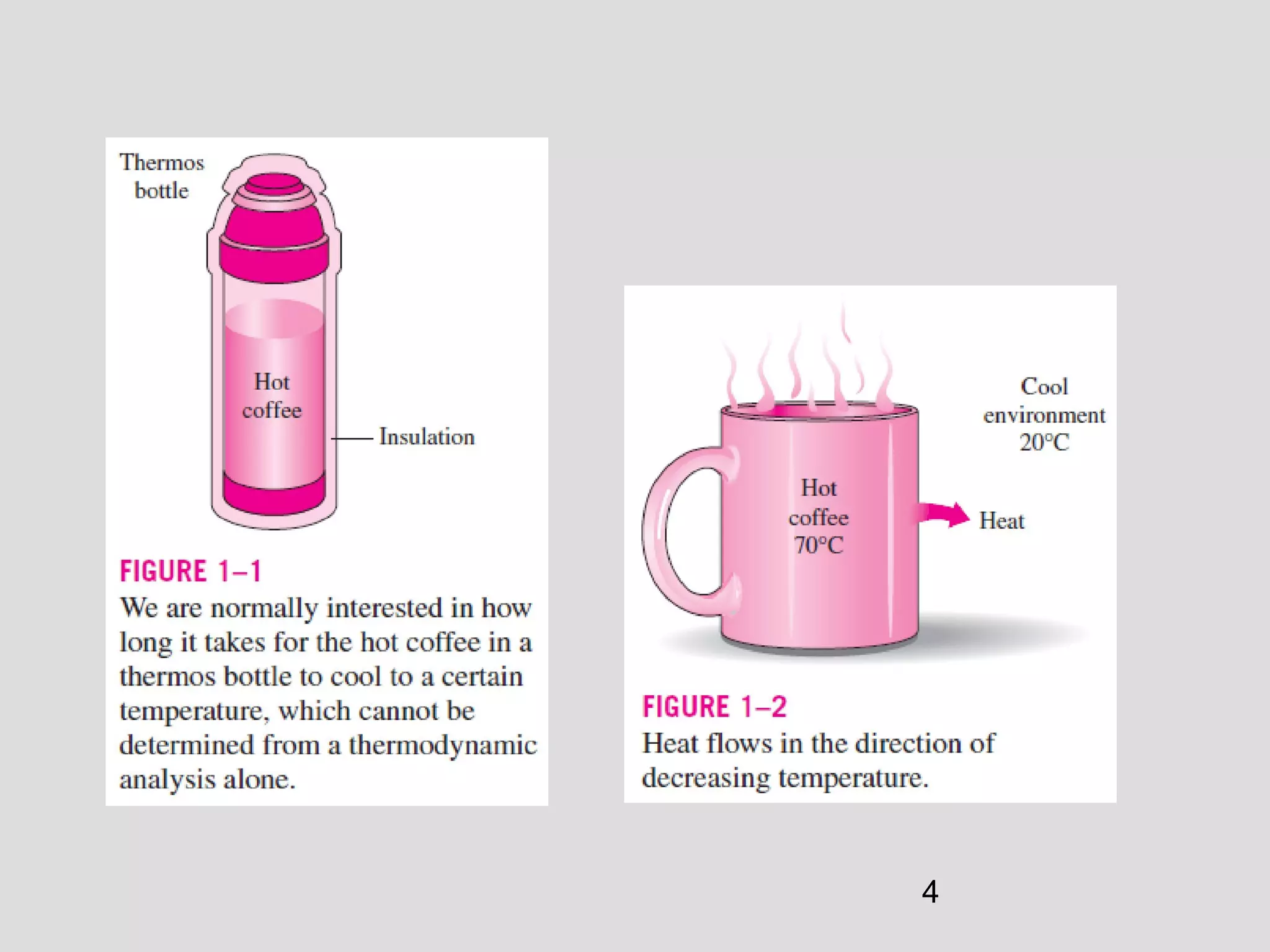
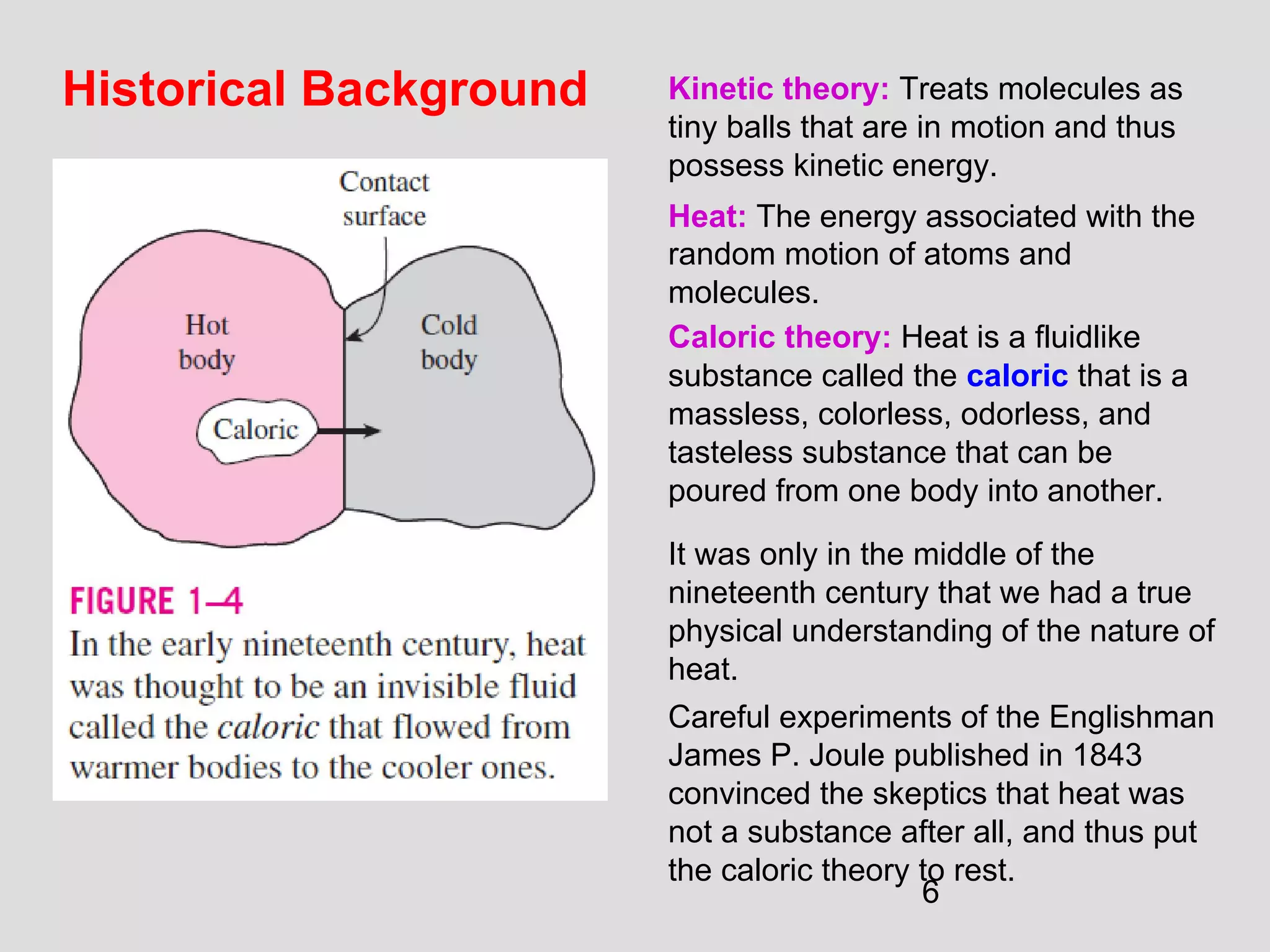
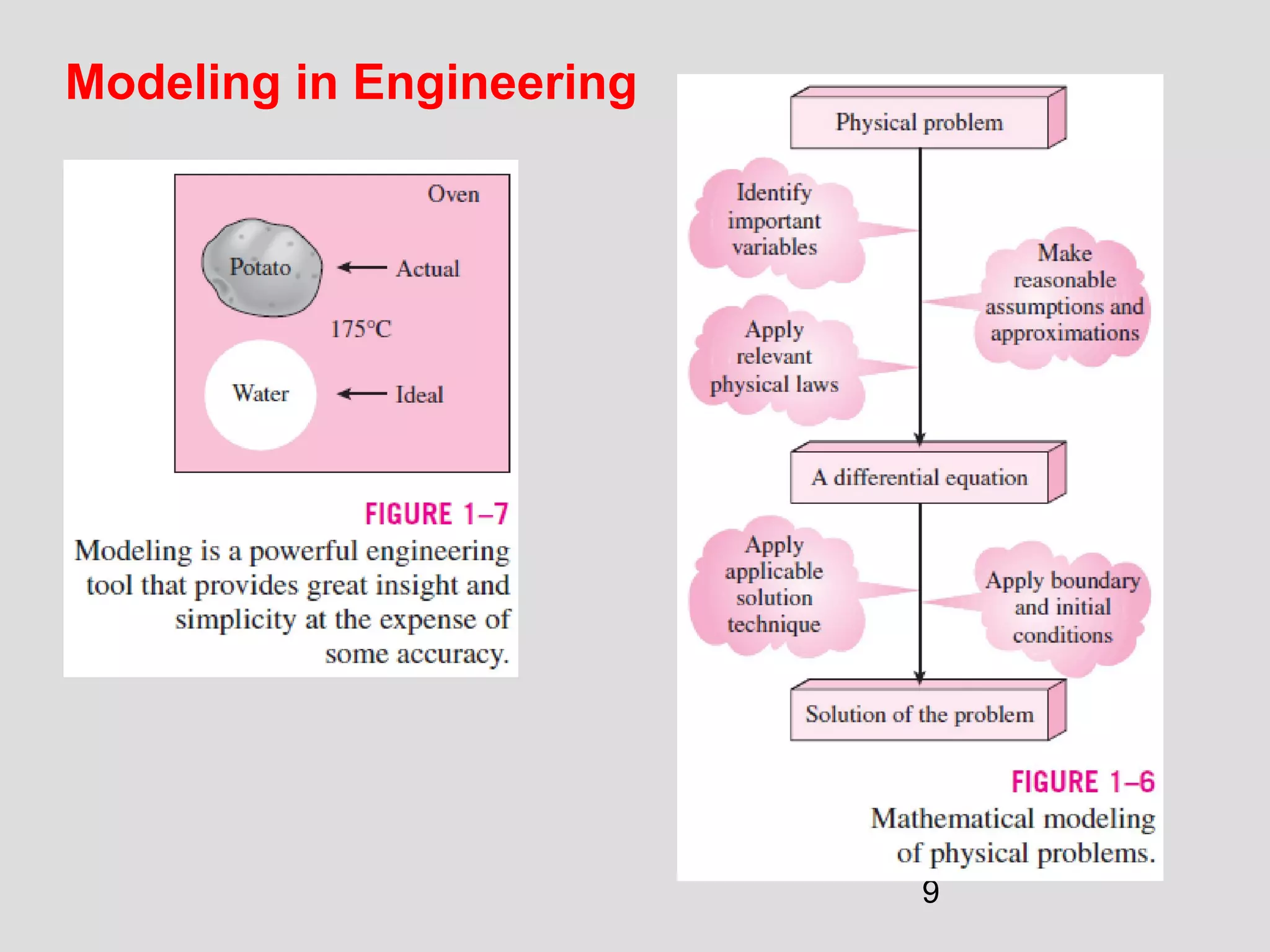
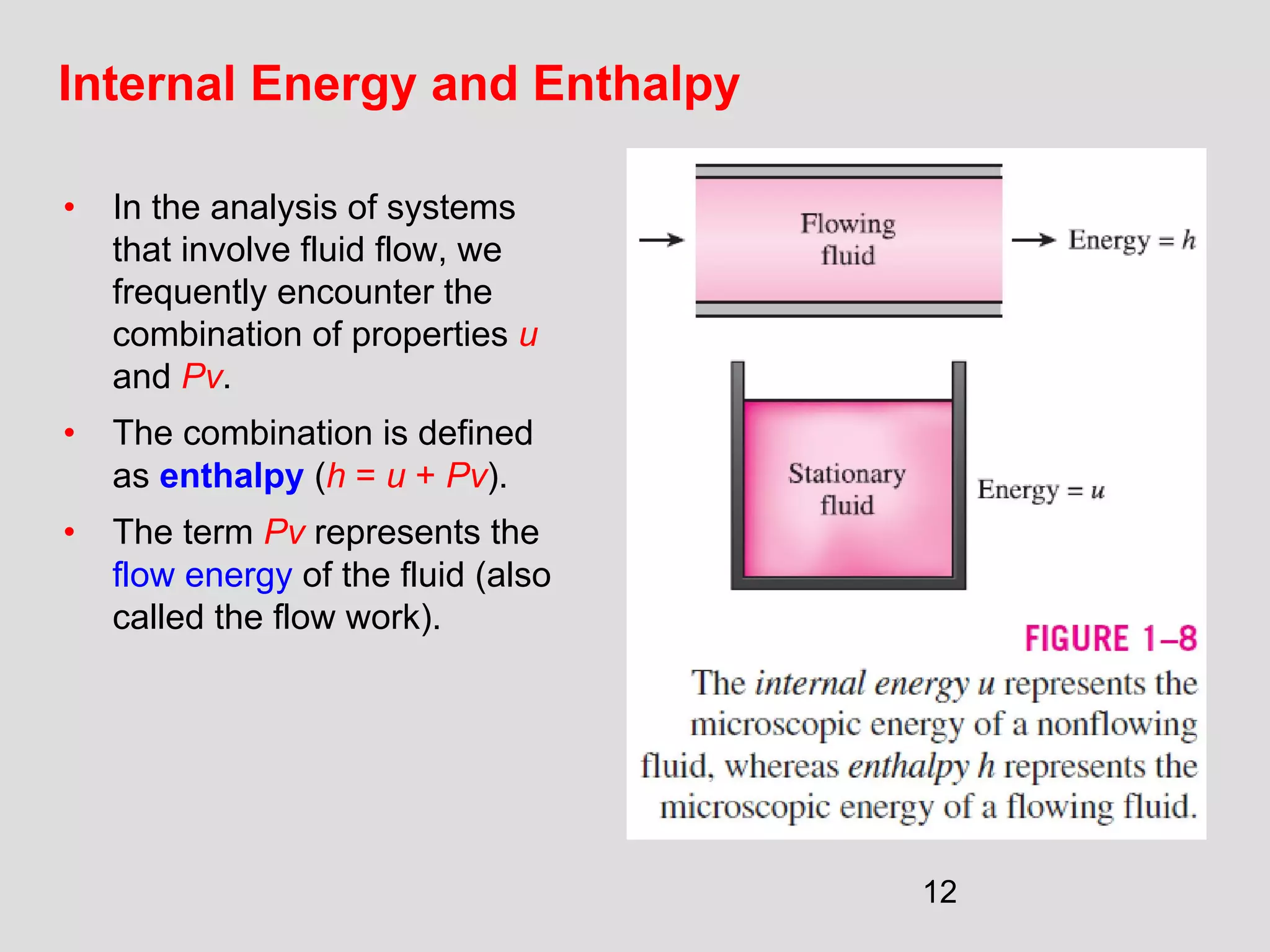
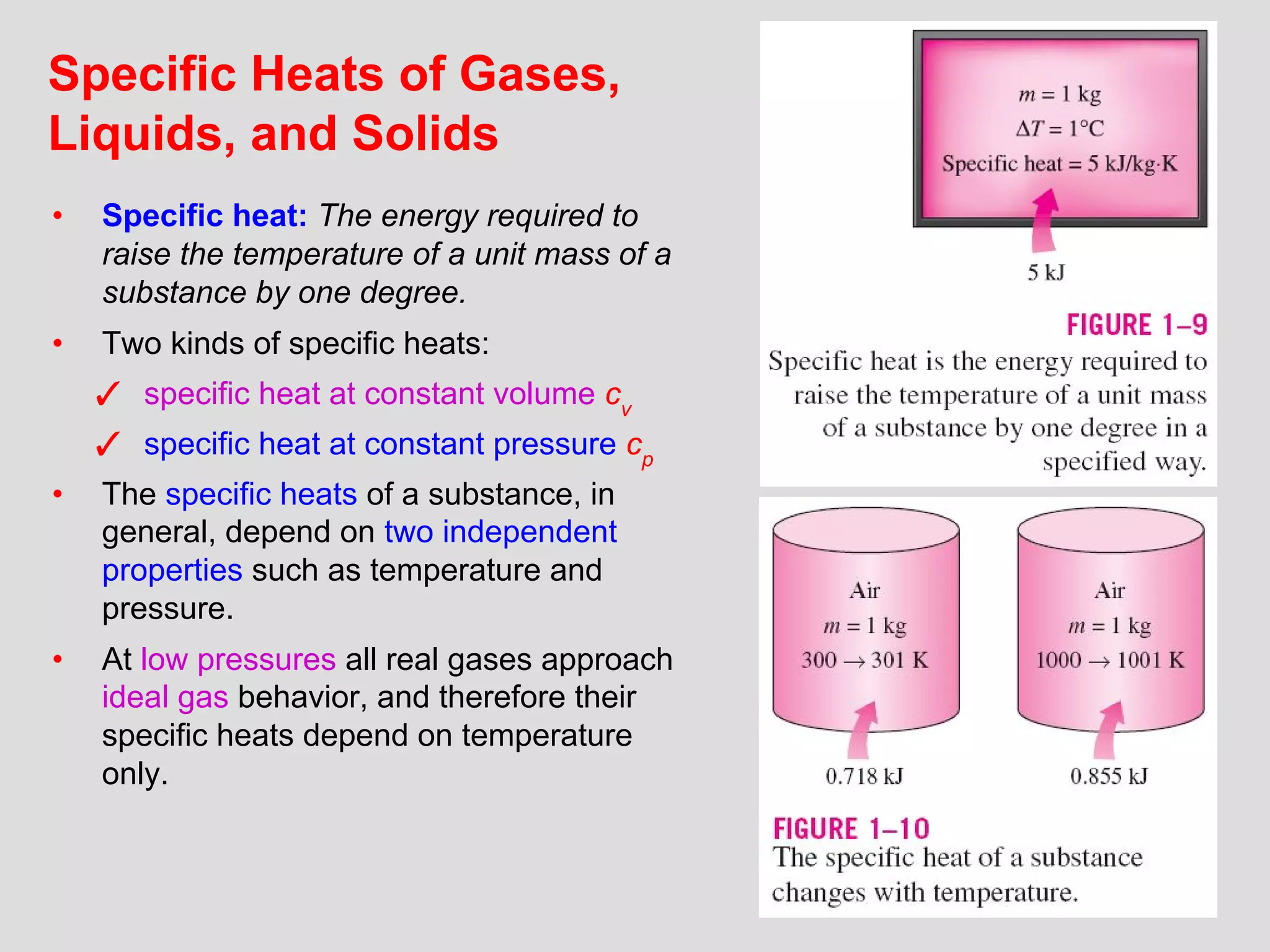
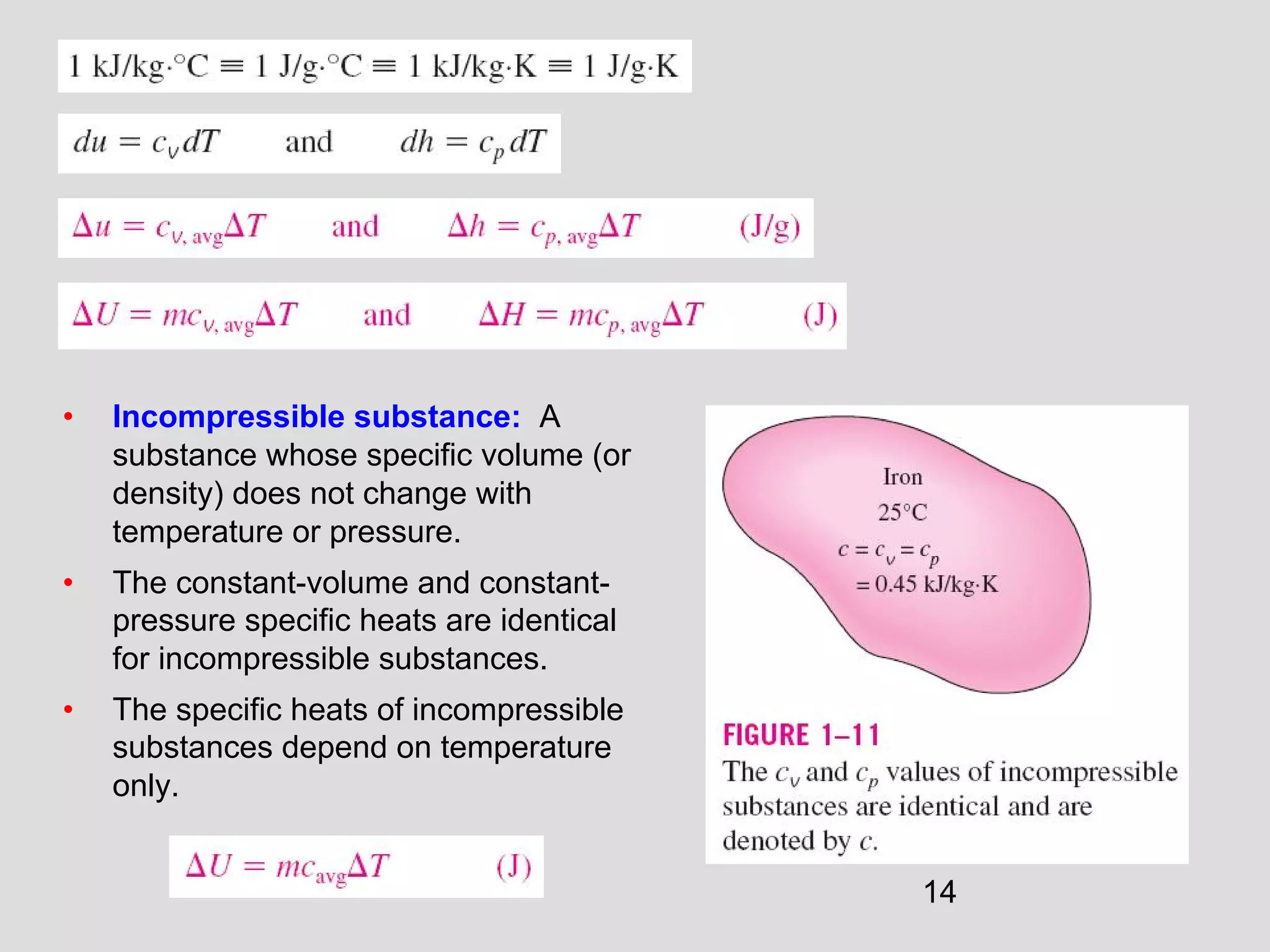
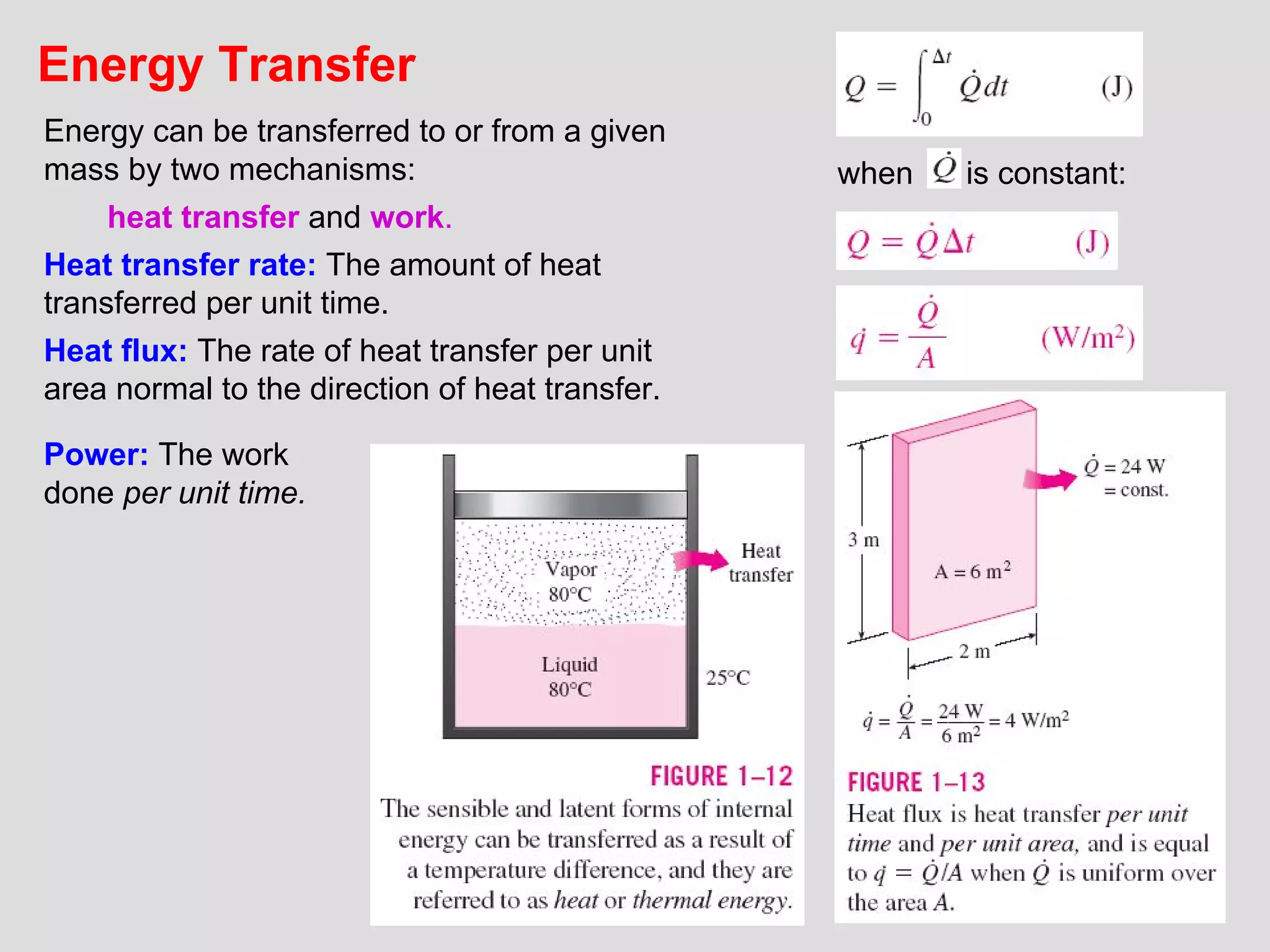
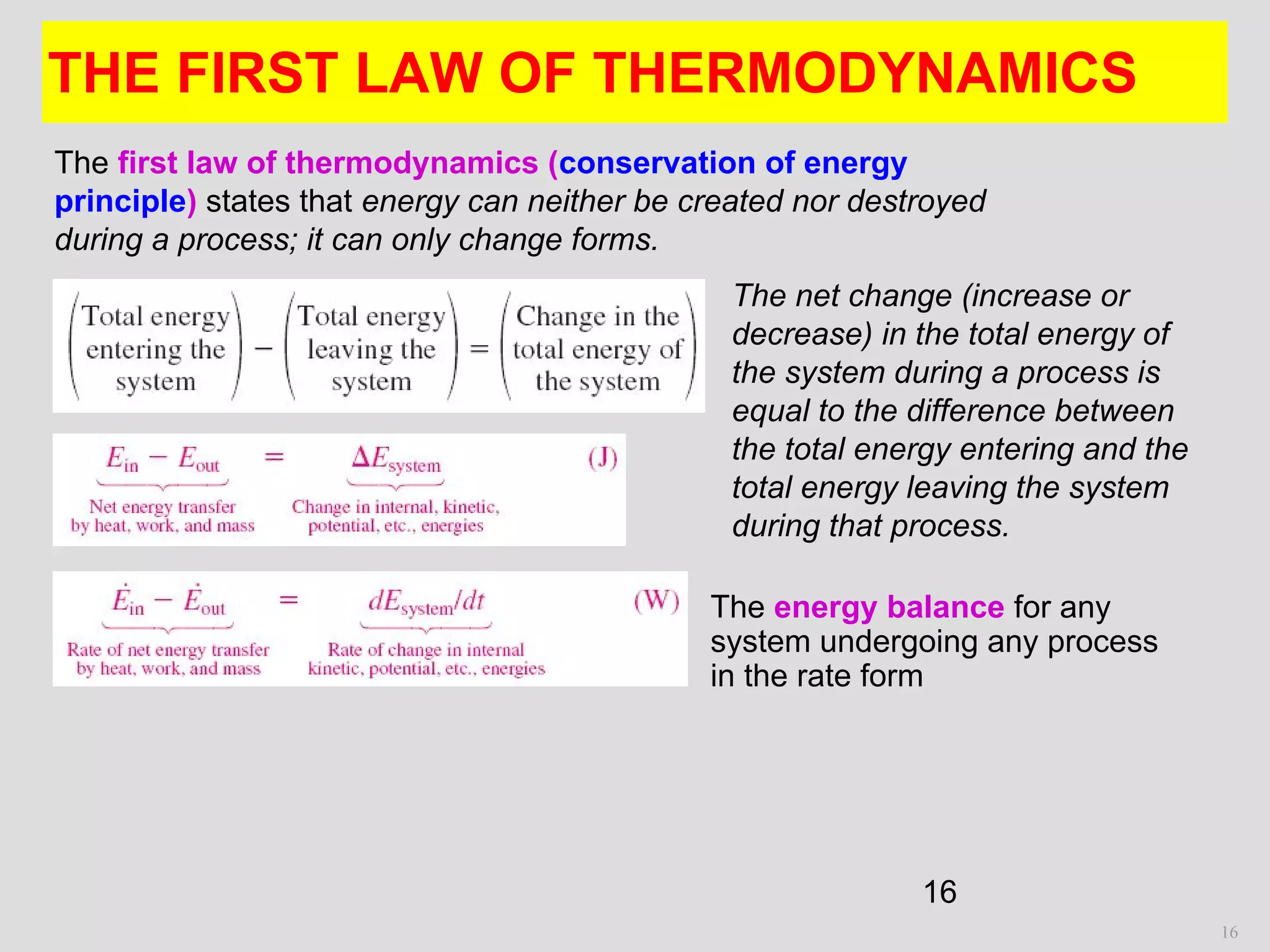
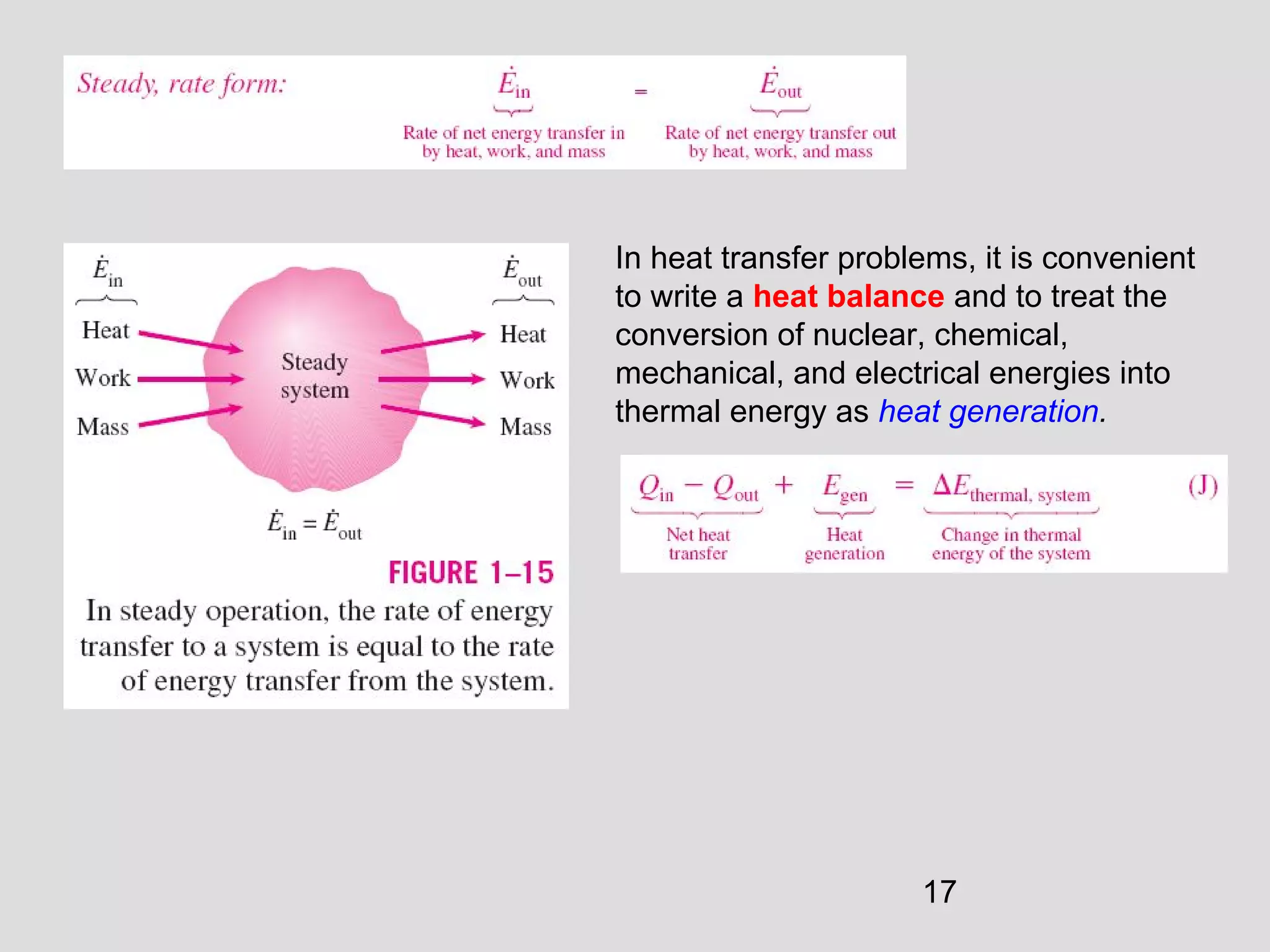
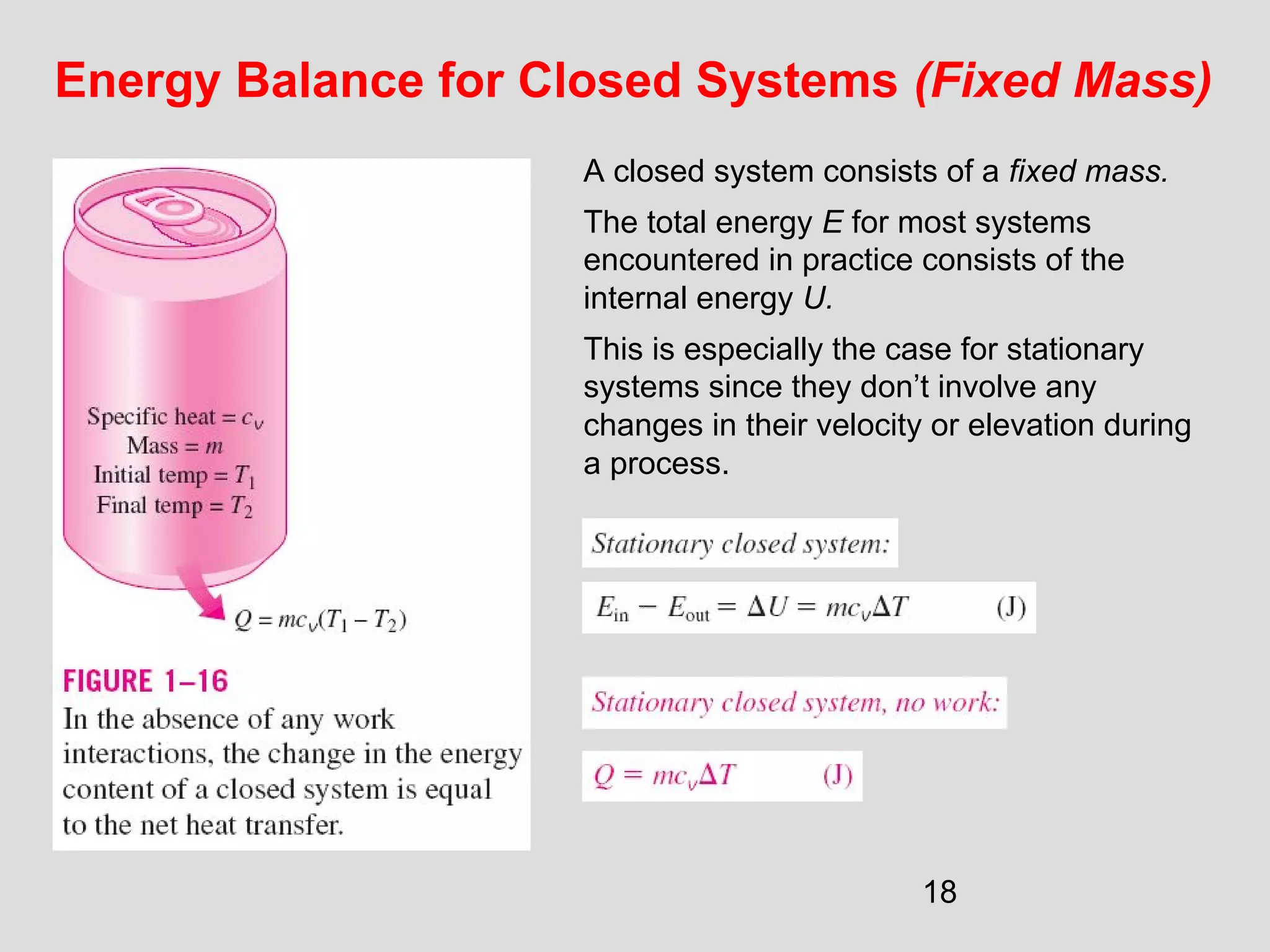
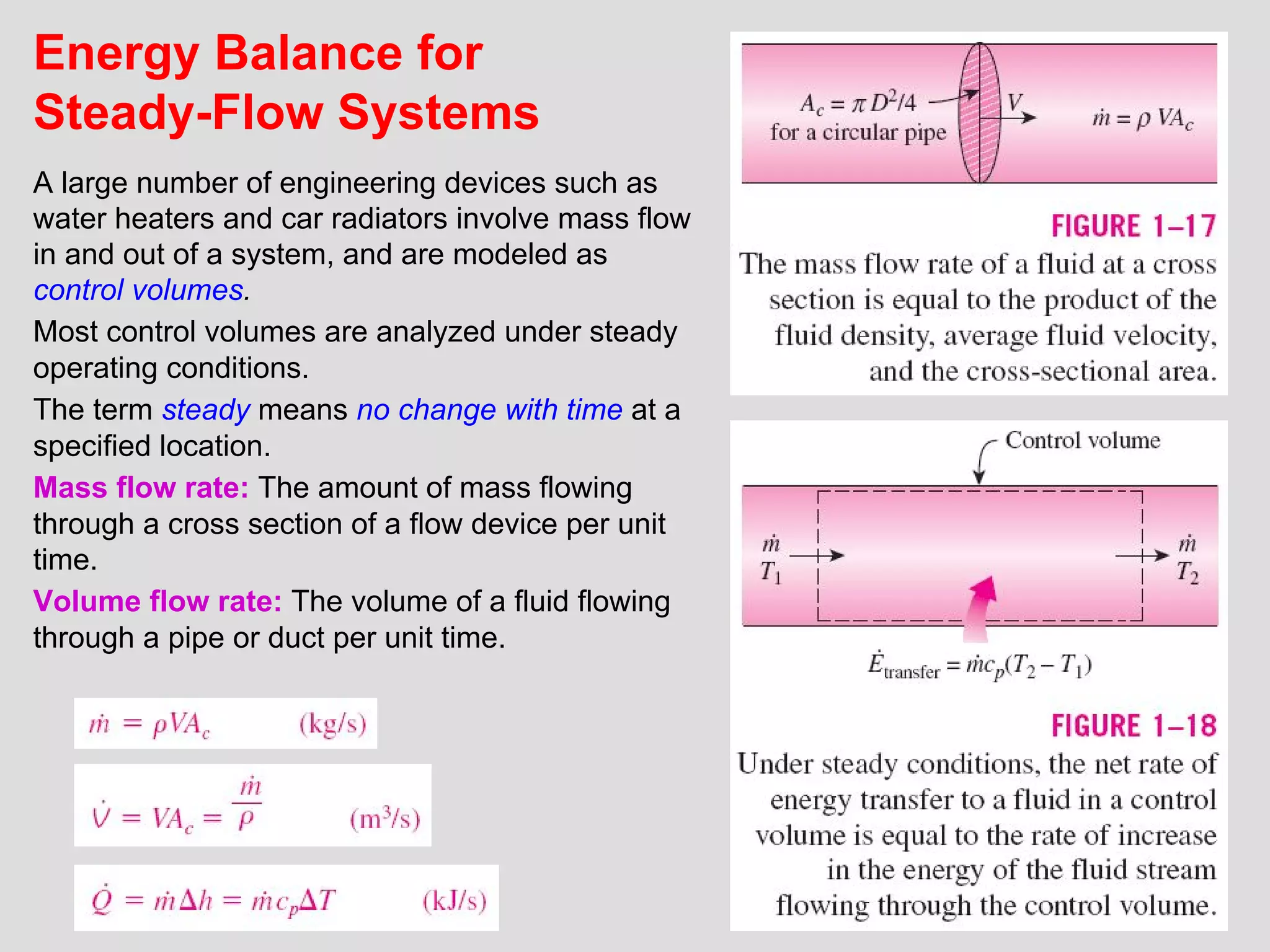
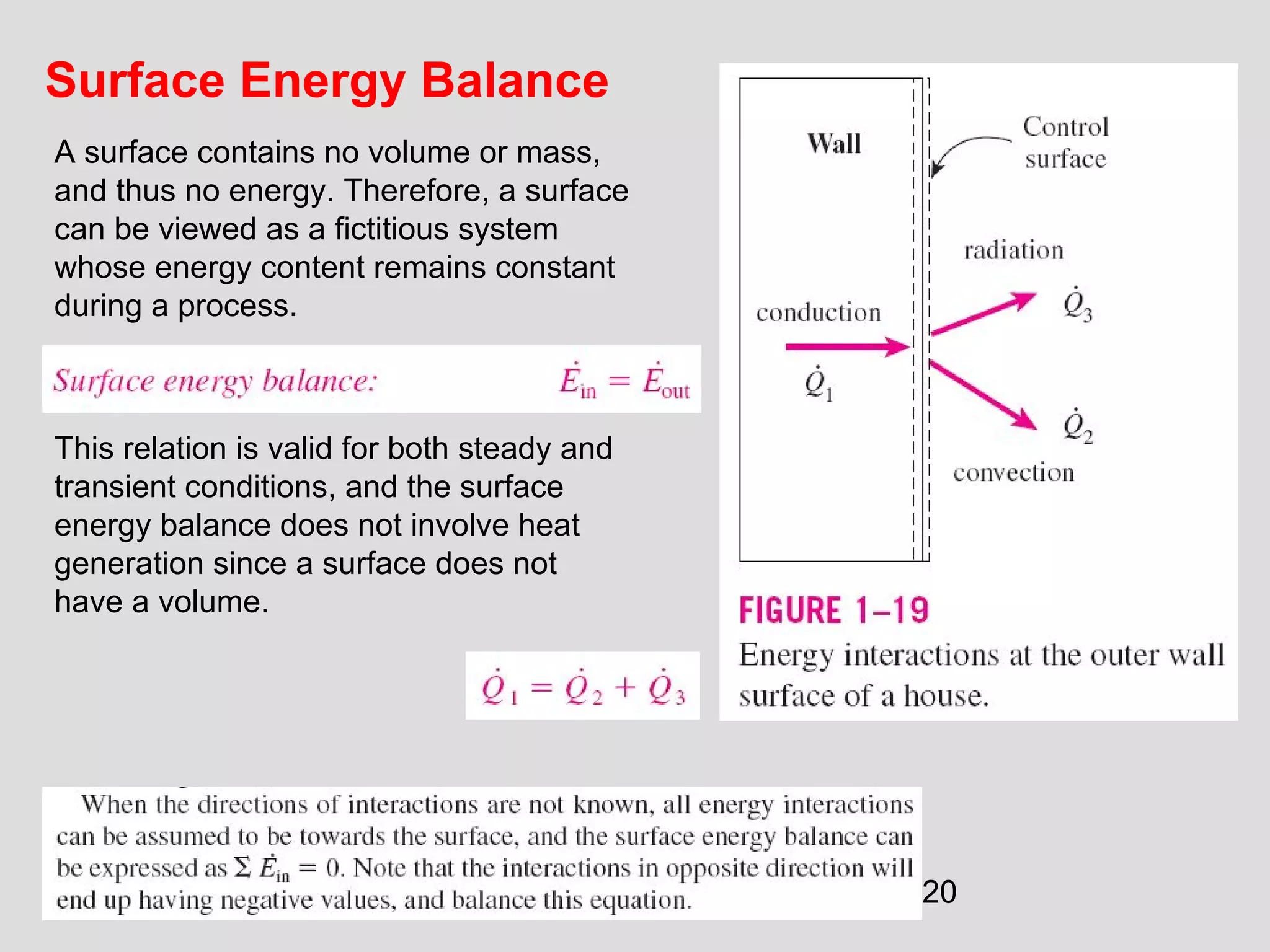
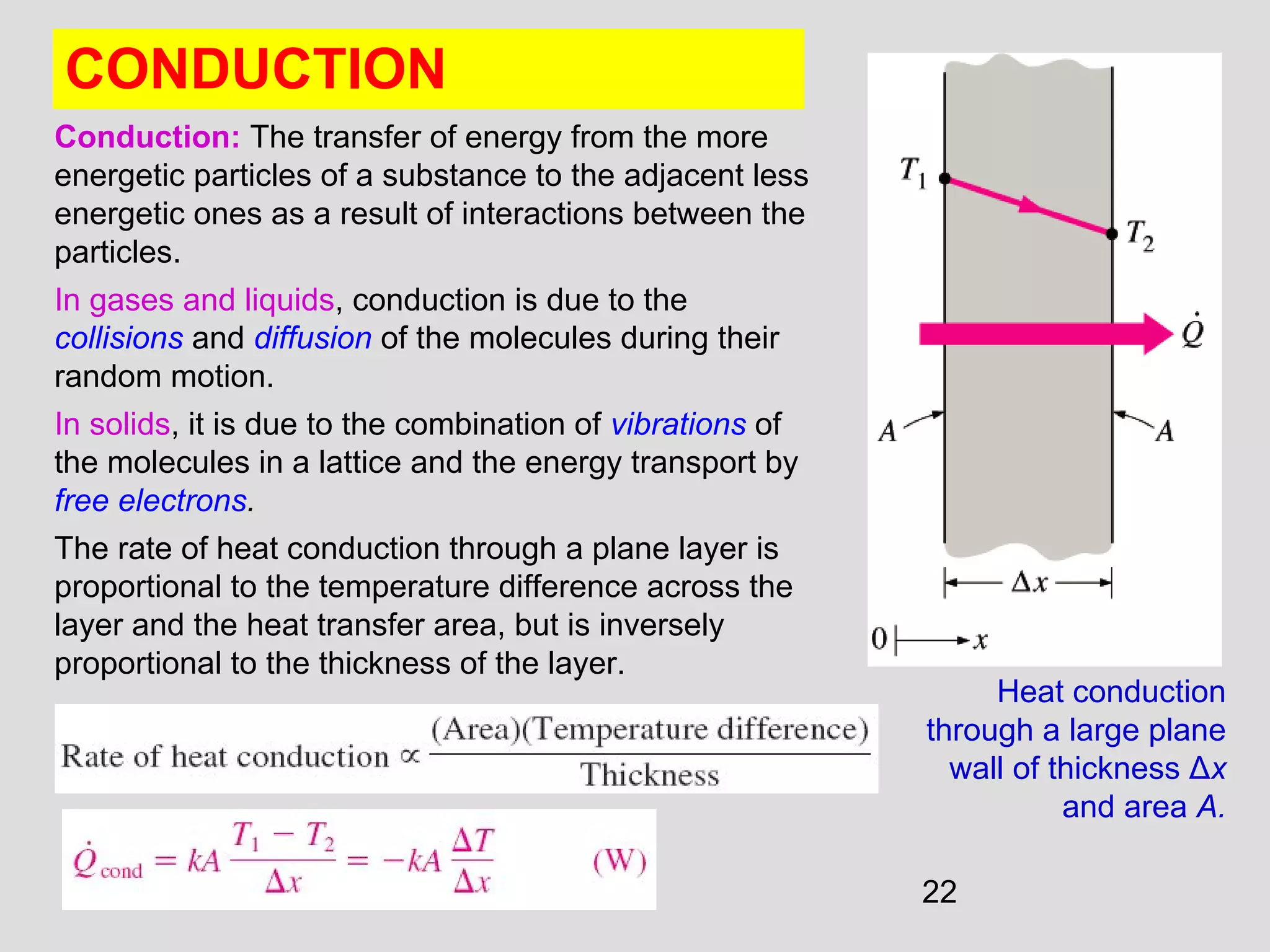
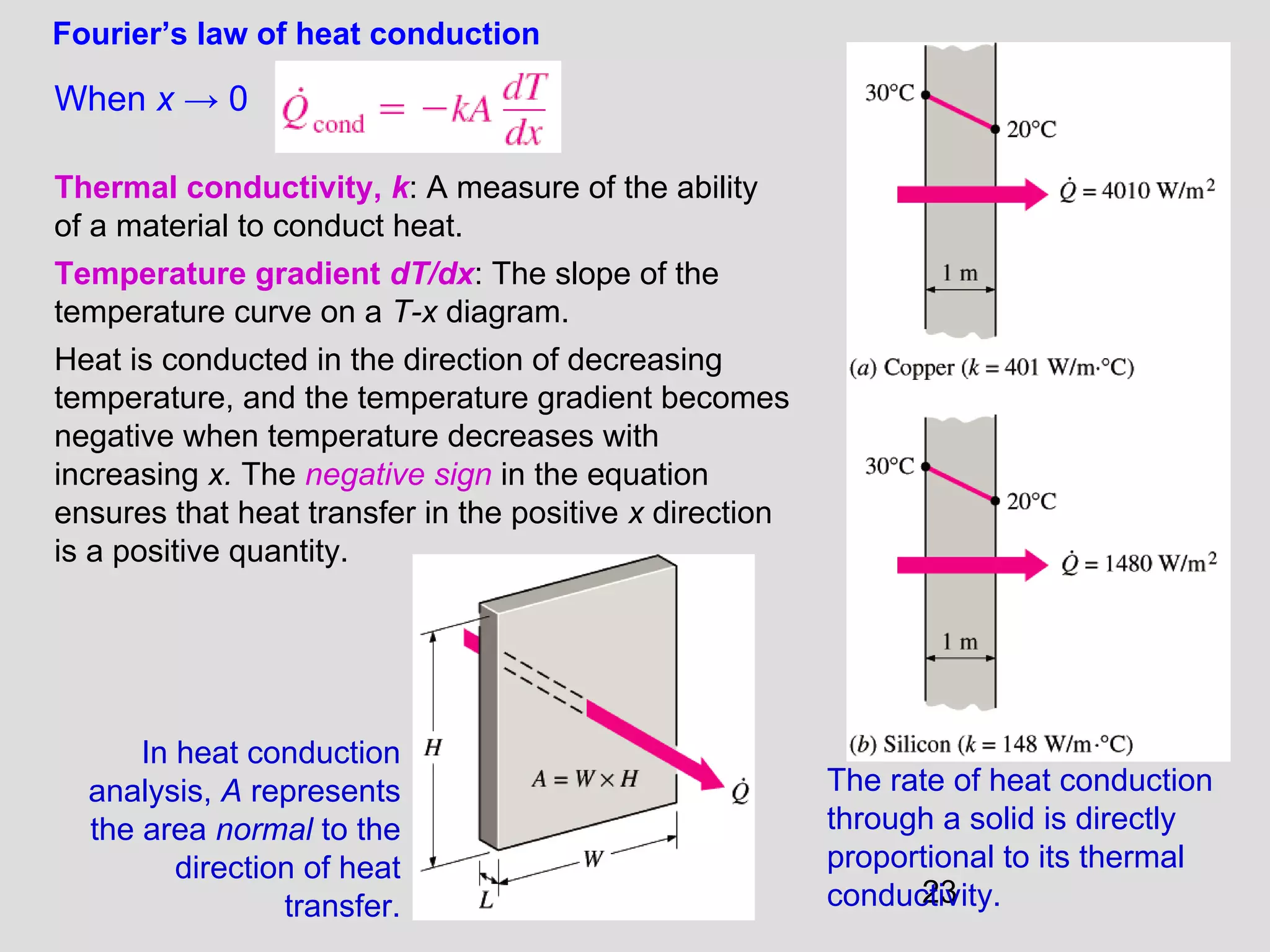
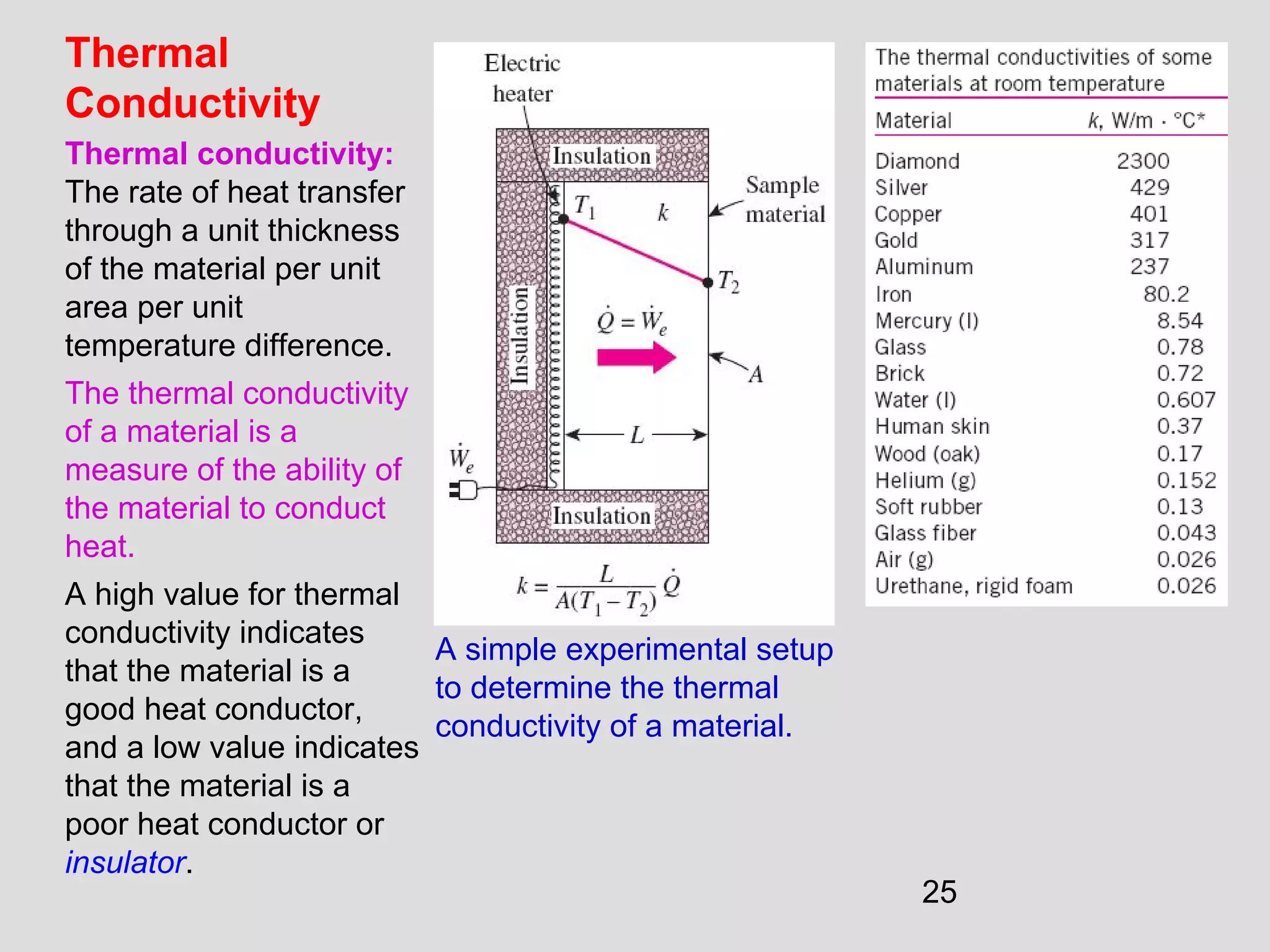
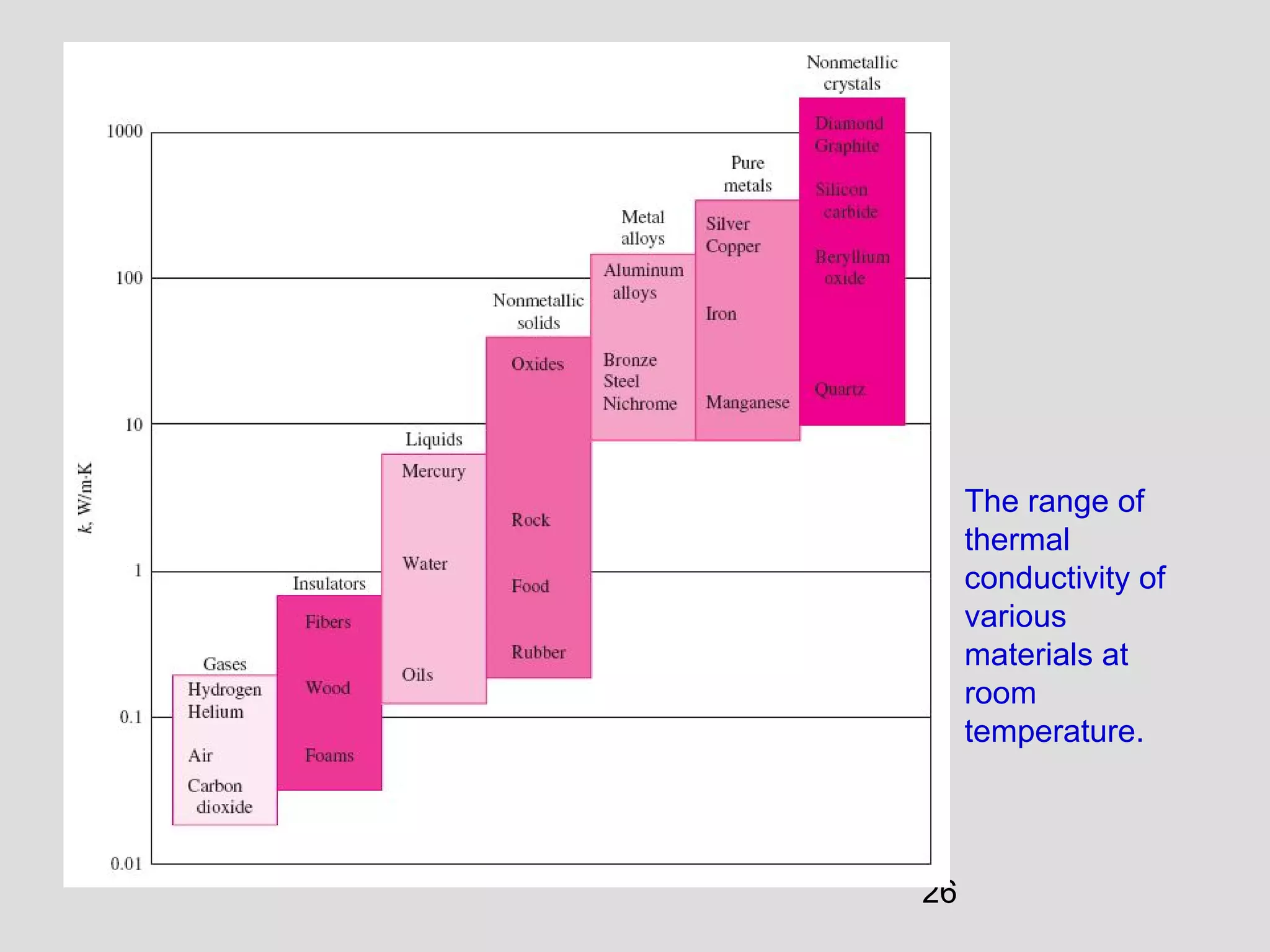
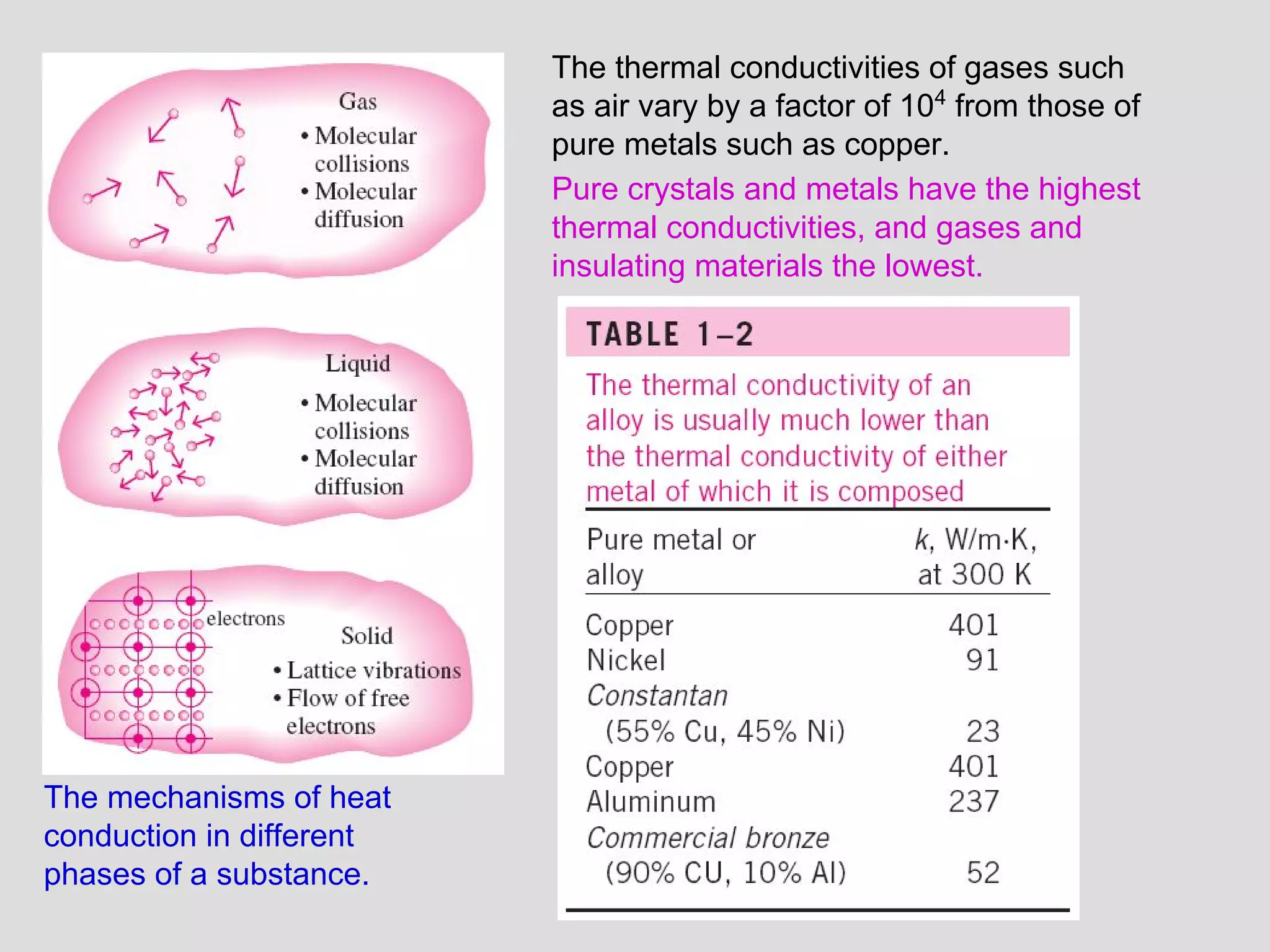
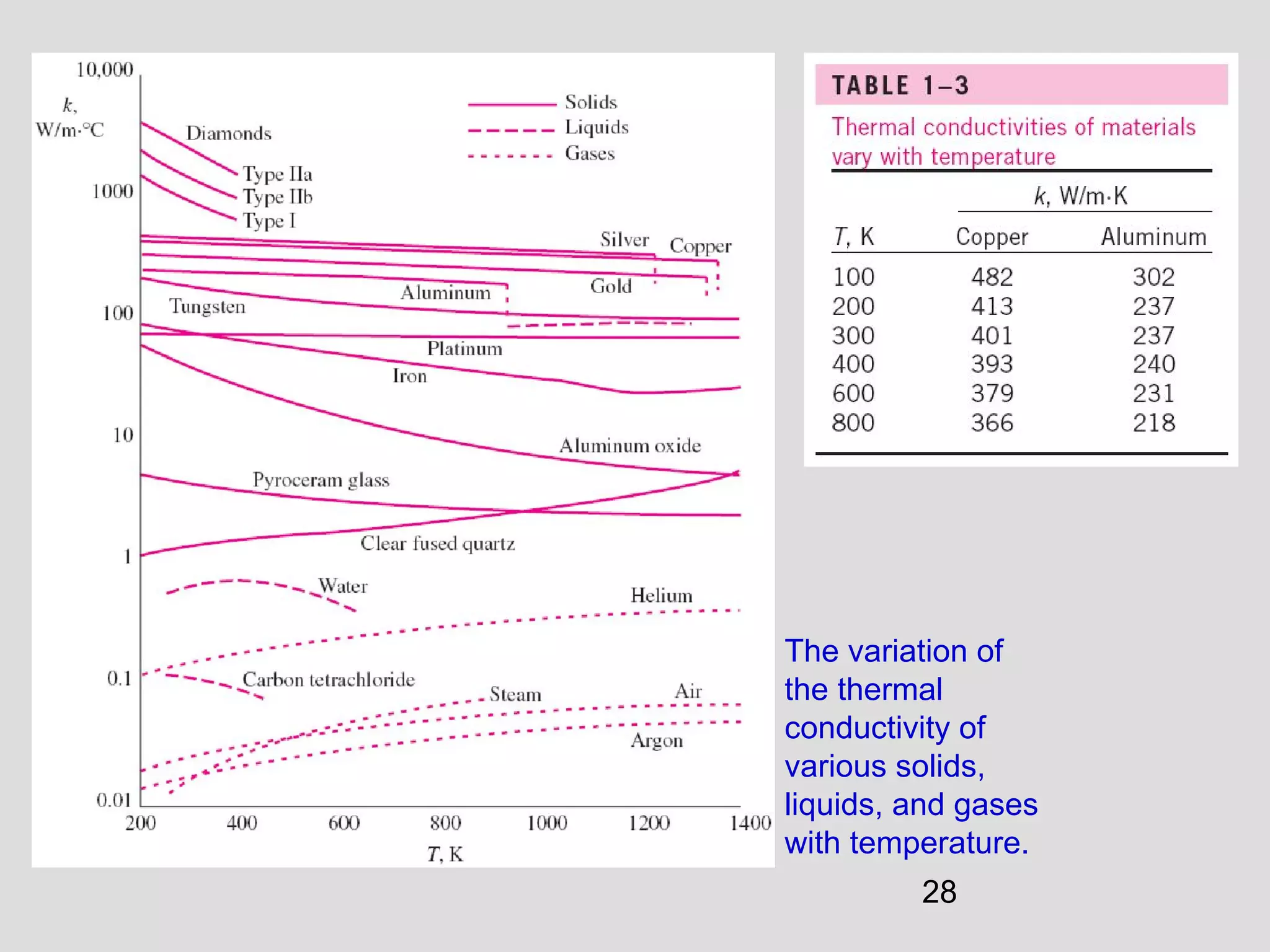
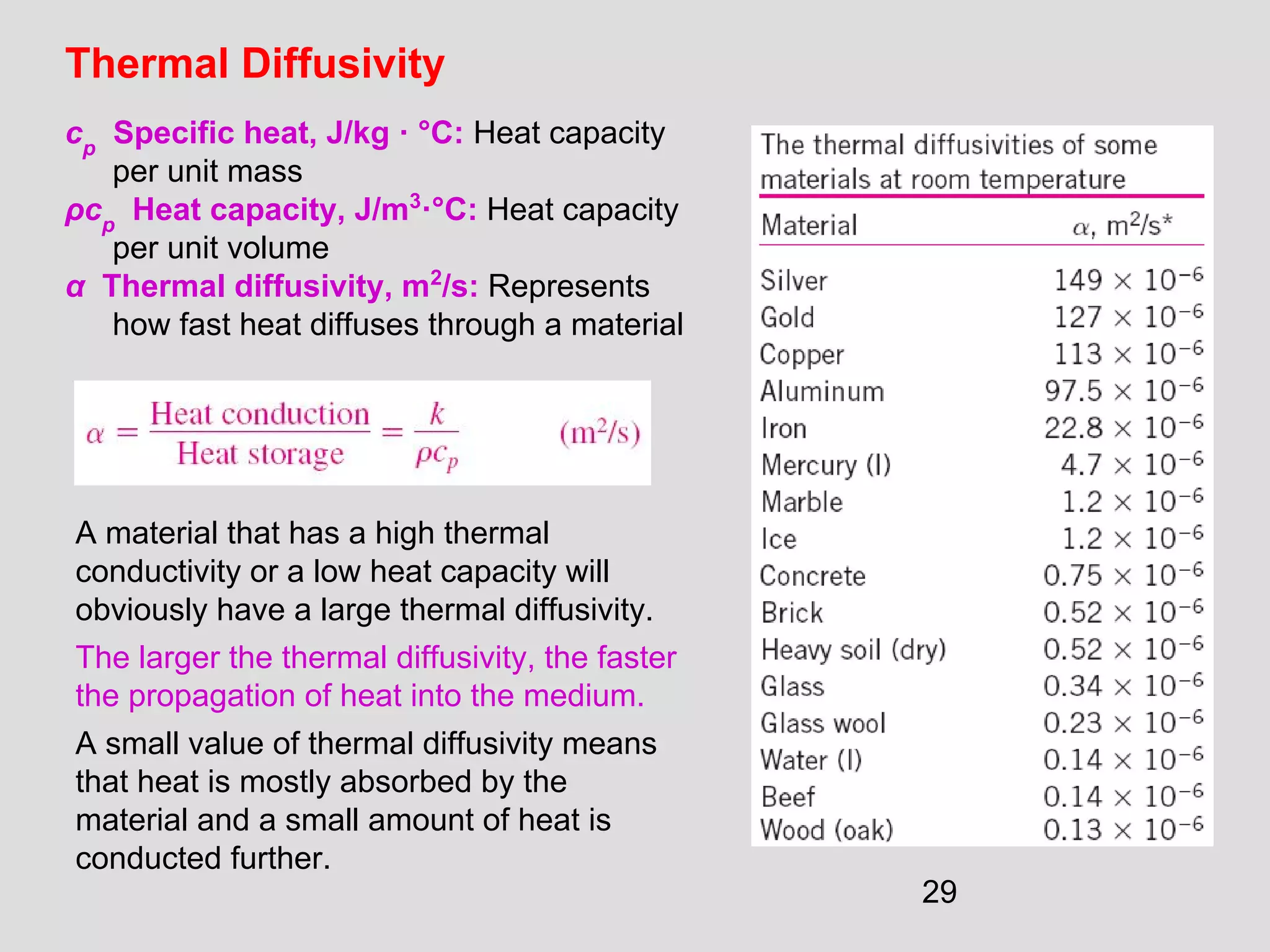
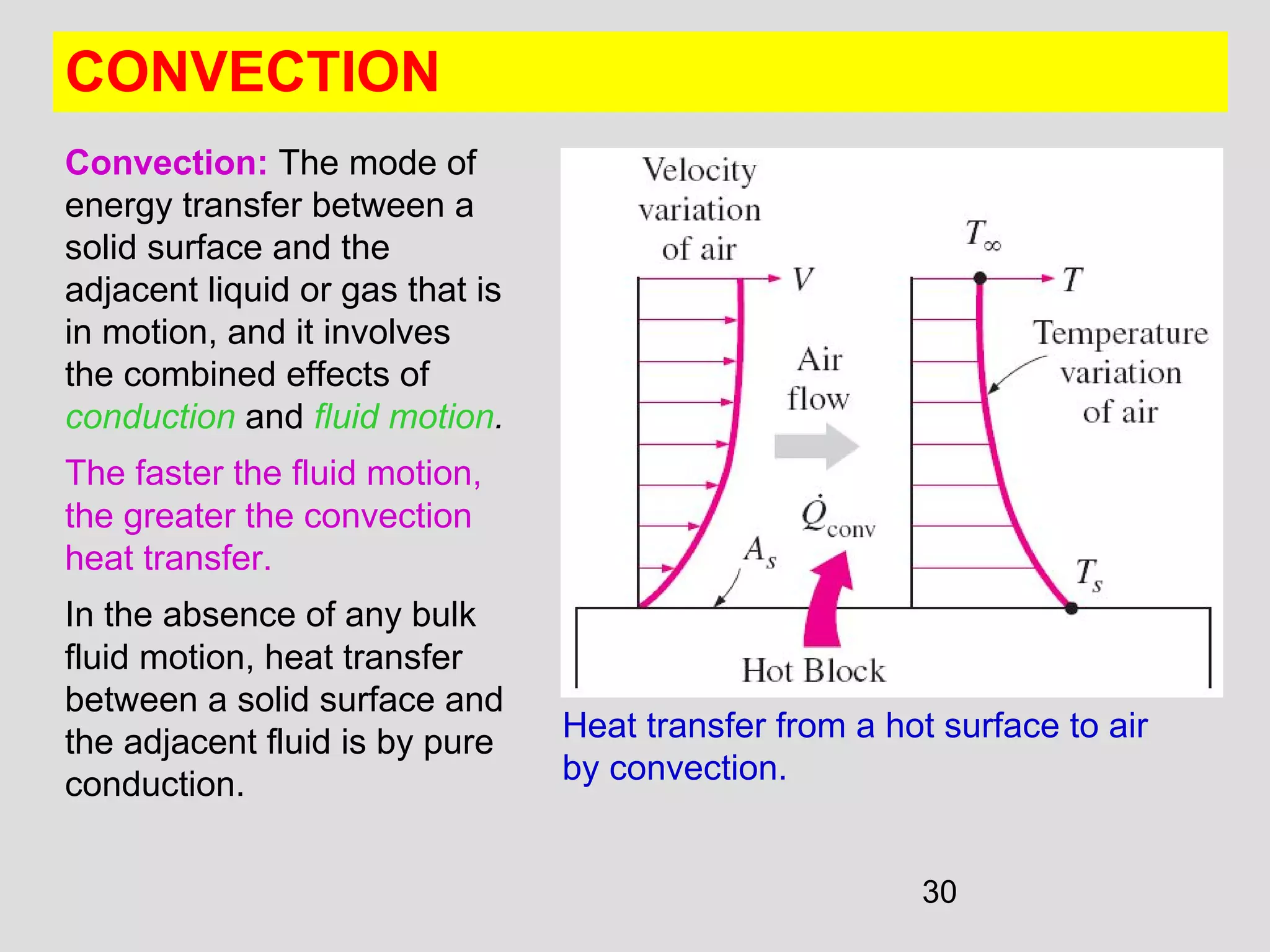
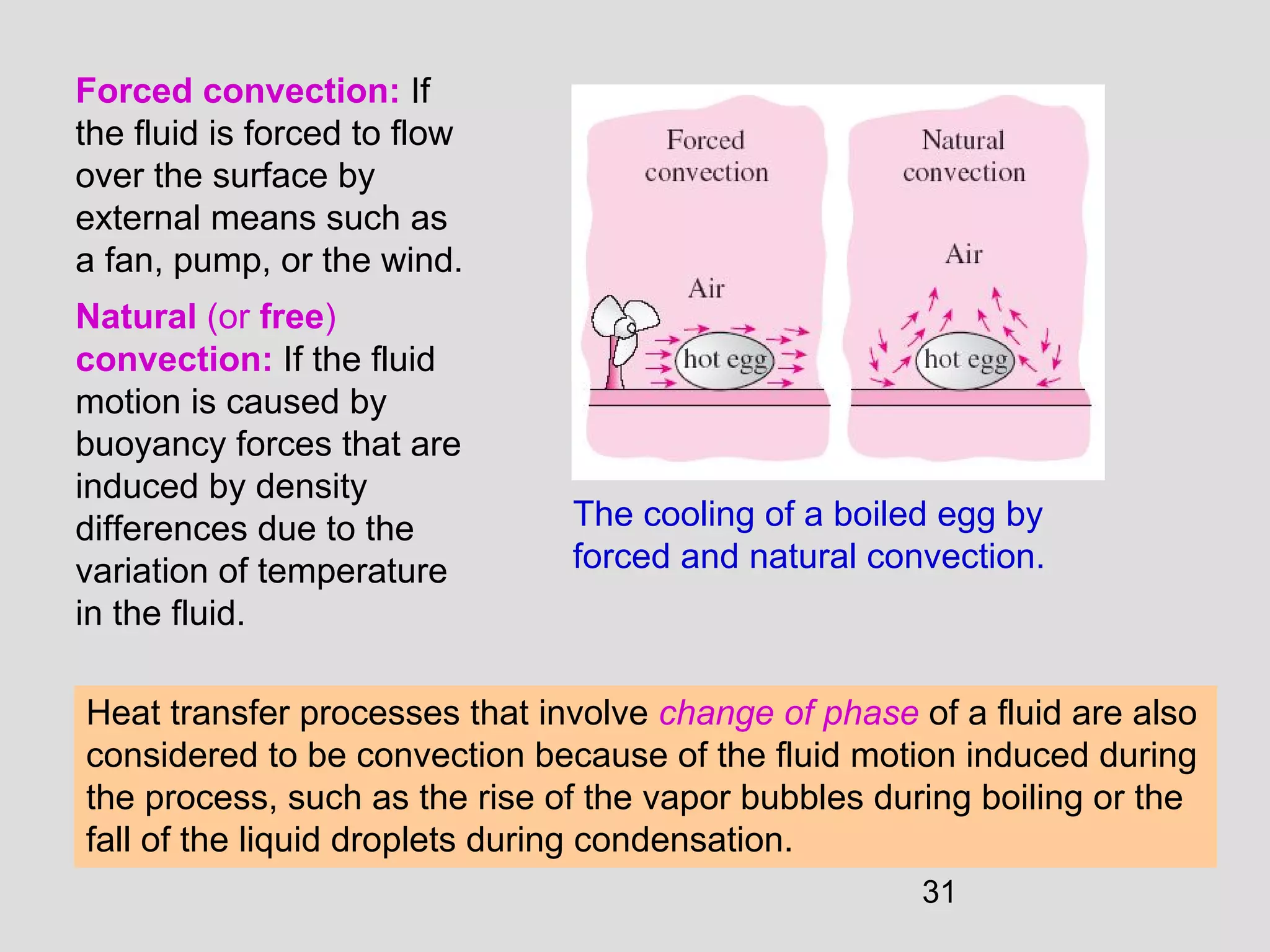
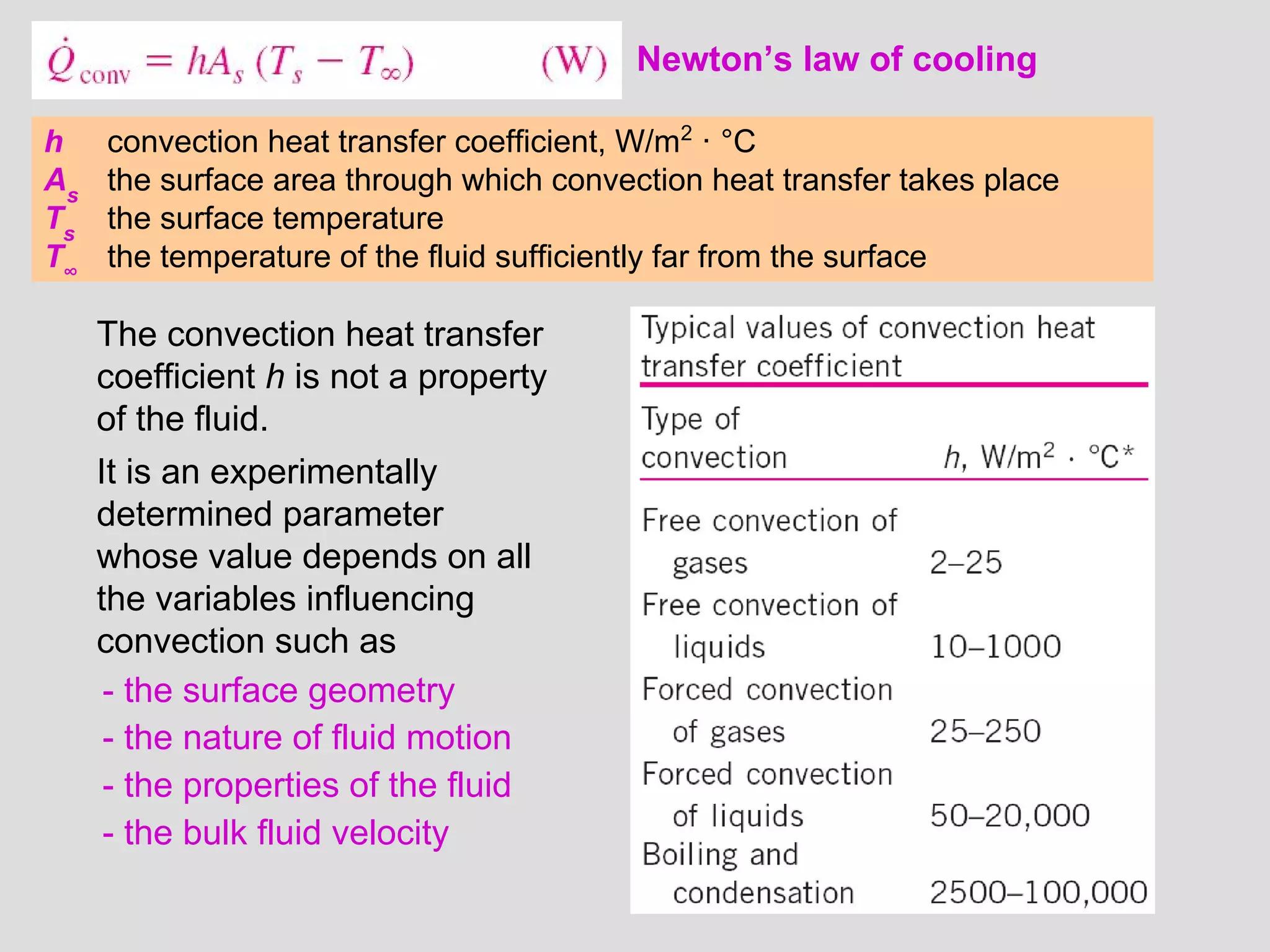
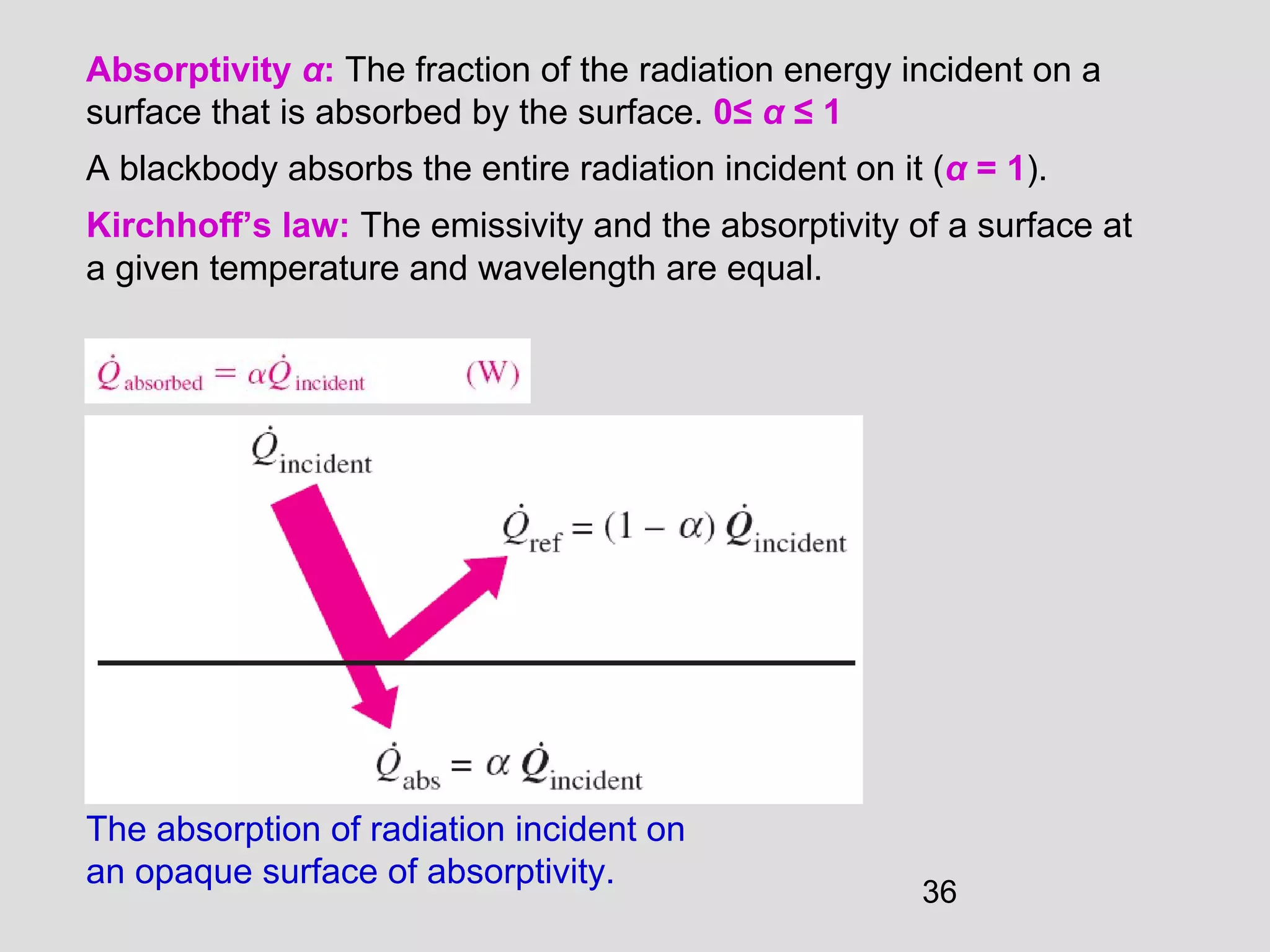
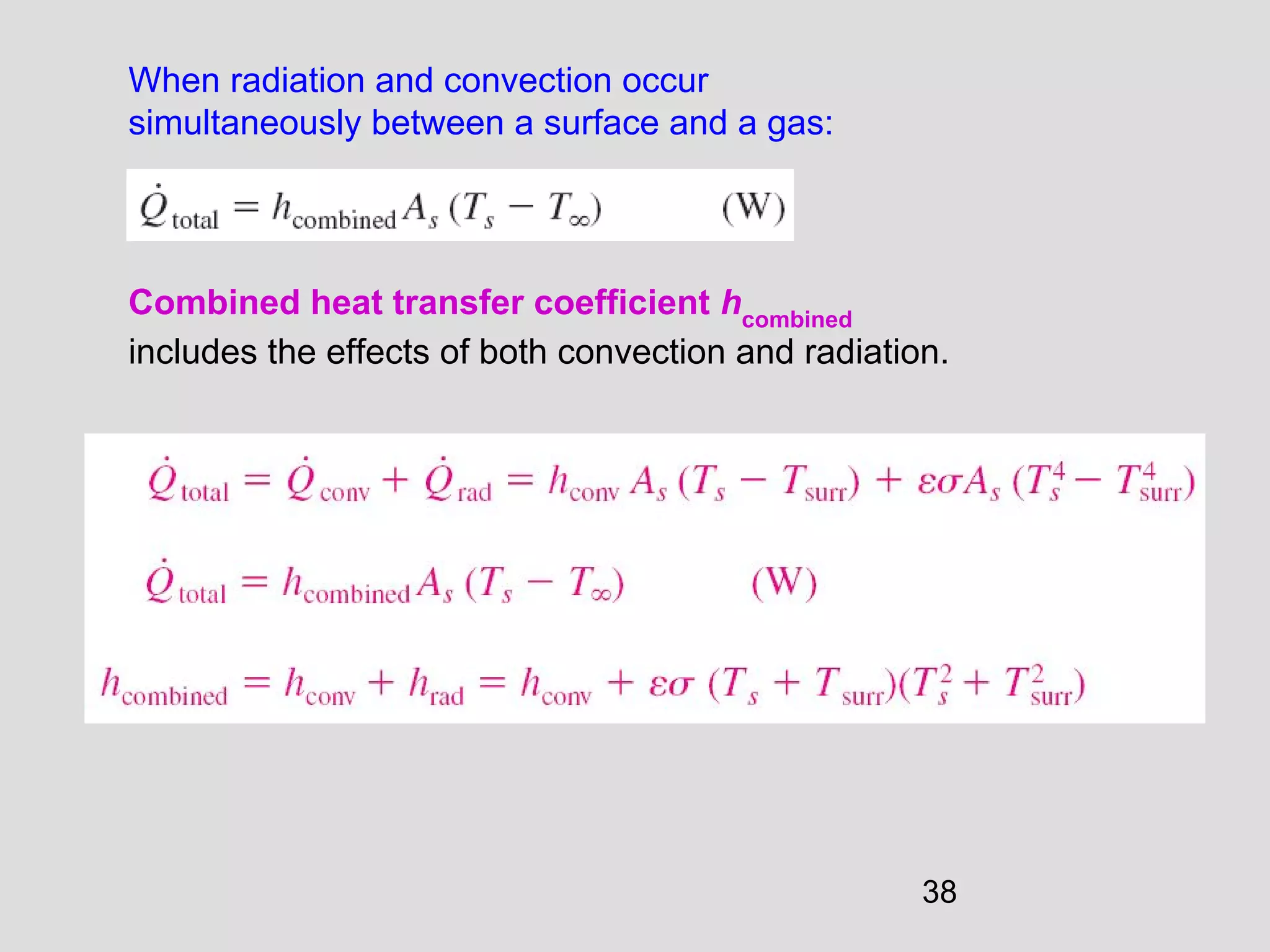
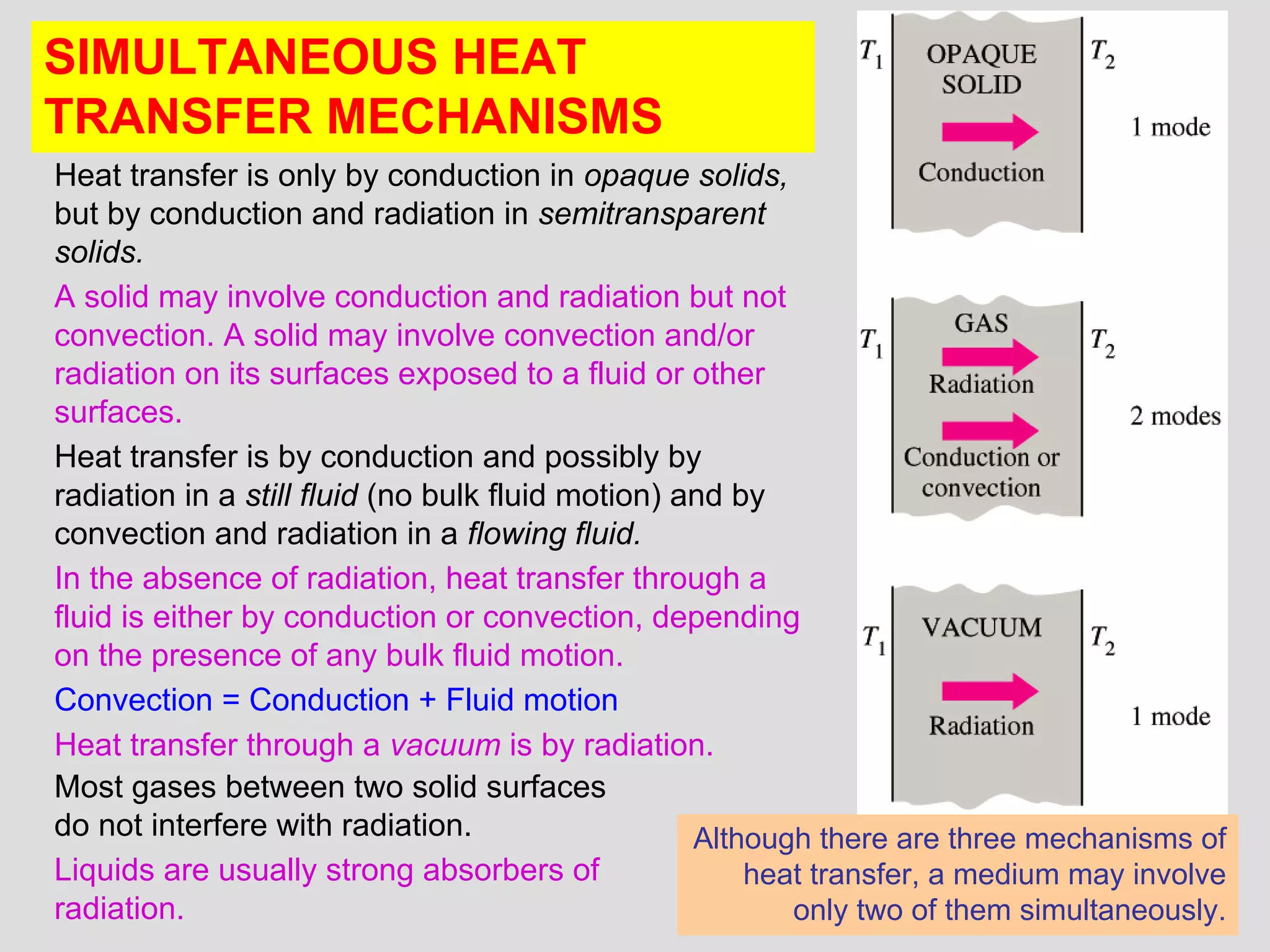
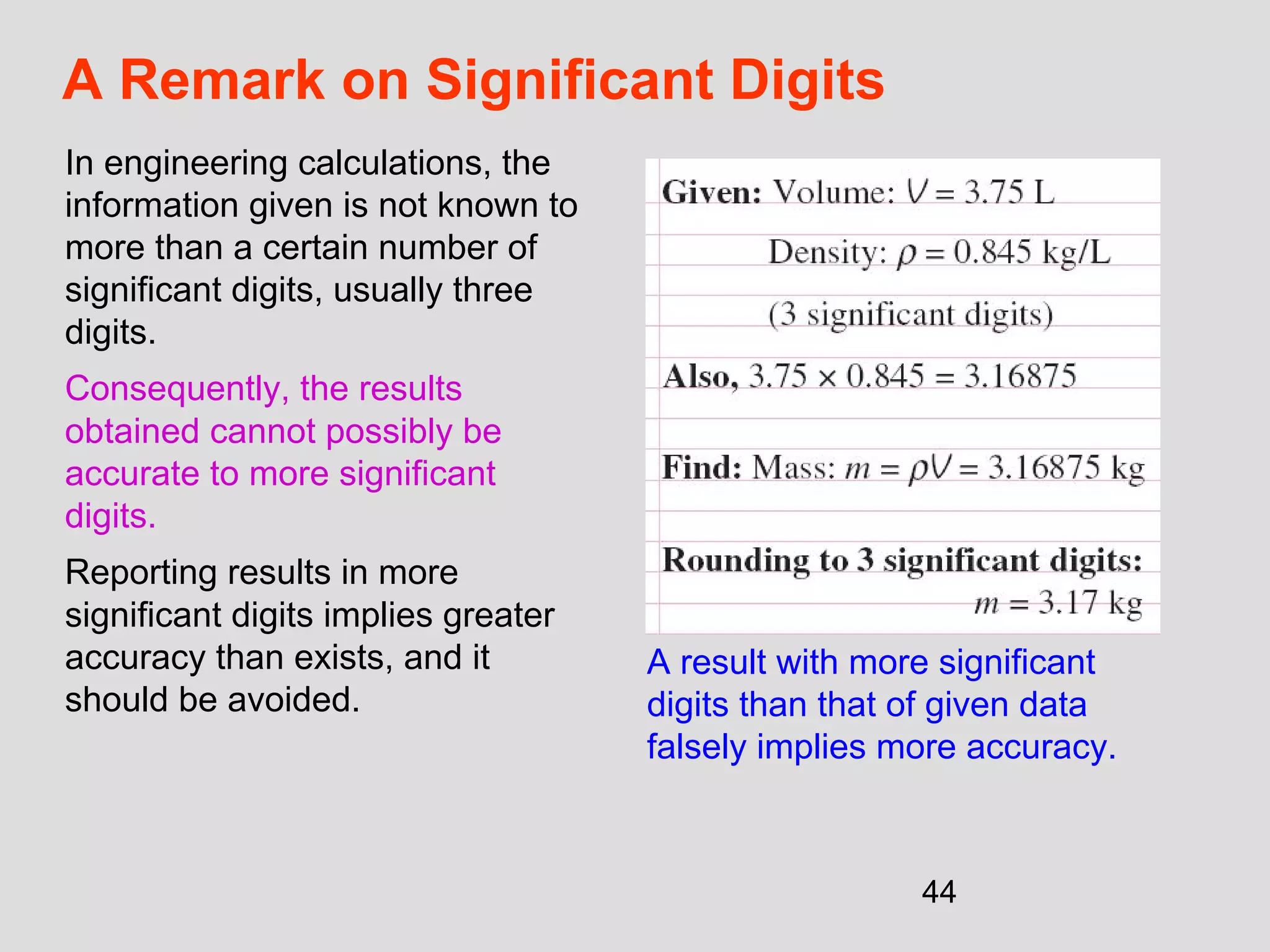
This document provides an introduction to heat transfer and thermodynamics concepts. It discusses how heat transfer is related to thermodynamics and distinguishes between different forms of energy. The three main modes of heat transfer are conduction, convection and radiation. Heat is defined as the transfer of energy between two systems due to a temperature difference, and will flow from the higher temperature object to the lower temperature one. The document provides objectives and outlines concepts like thermal energy, mechanisms of heat transfer, Fourier's law of conduction and applications of heat transfer.