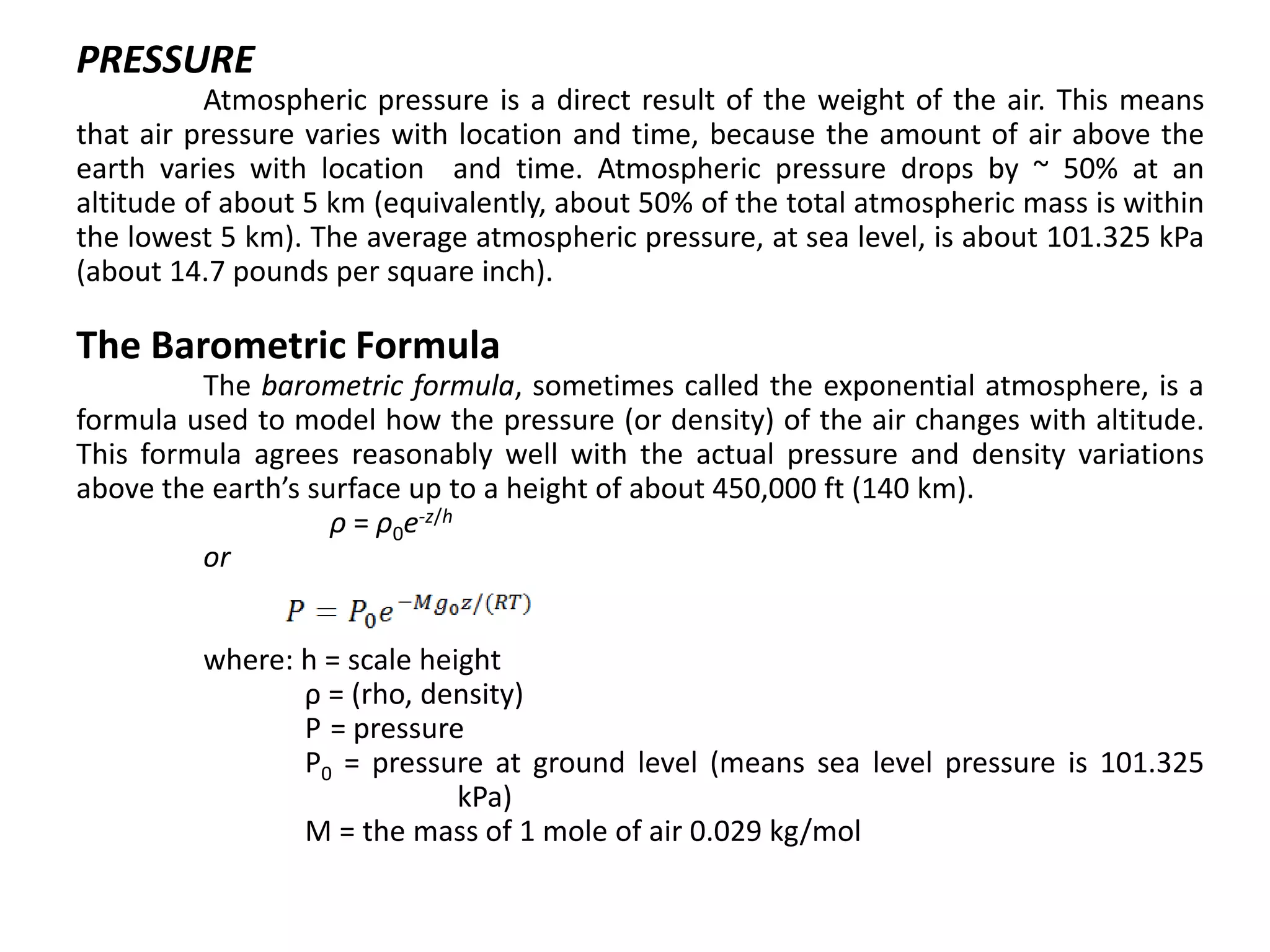
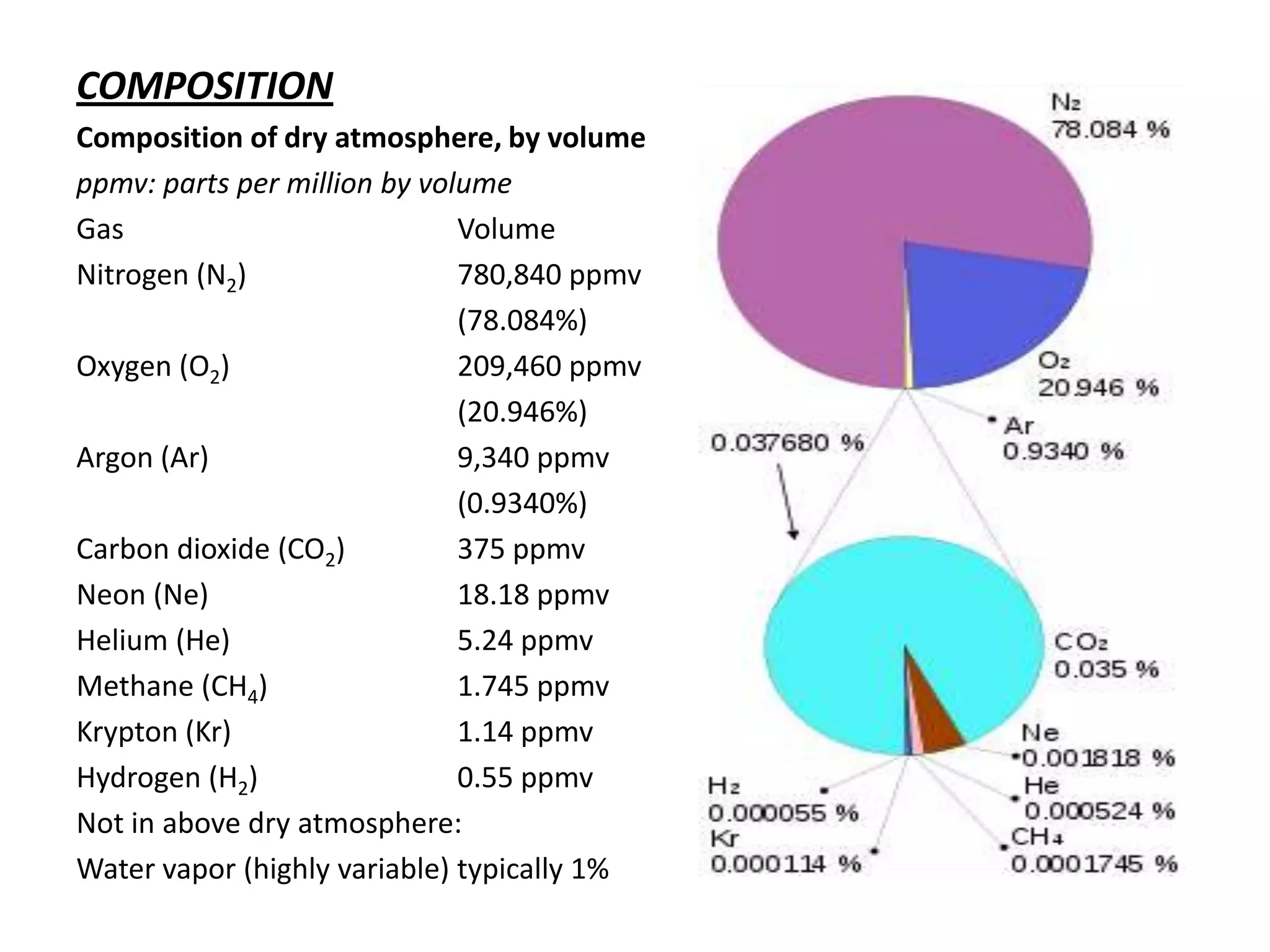
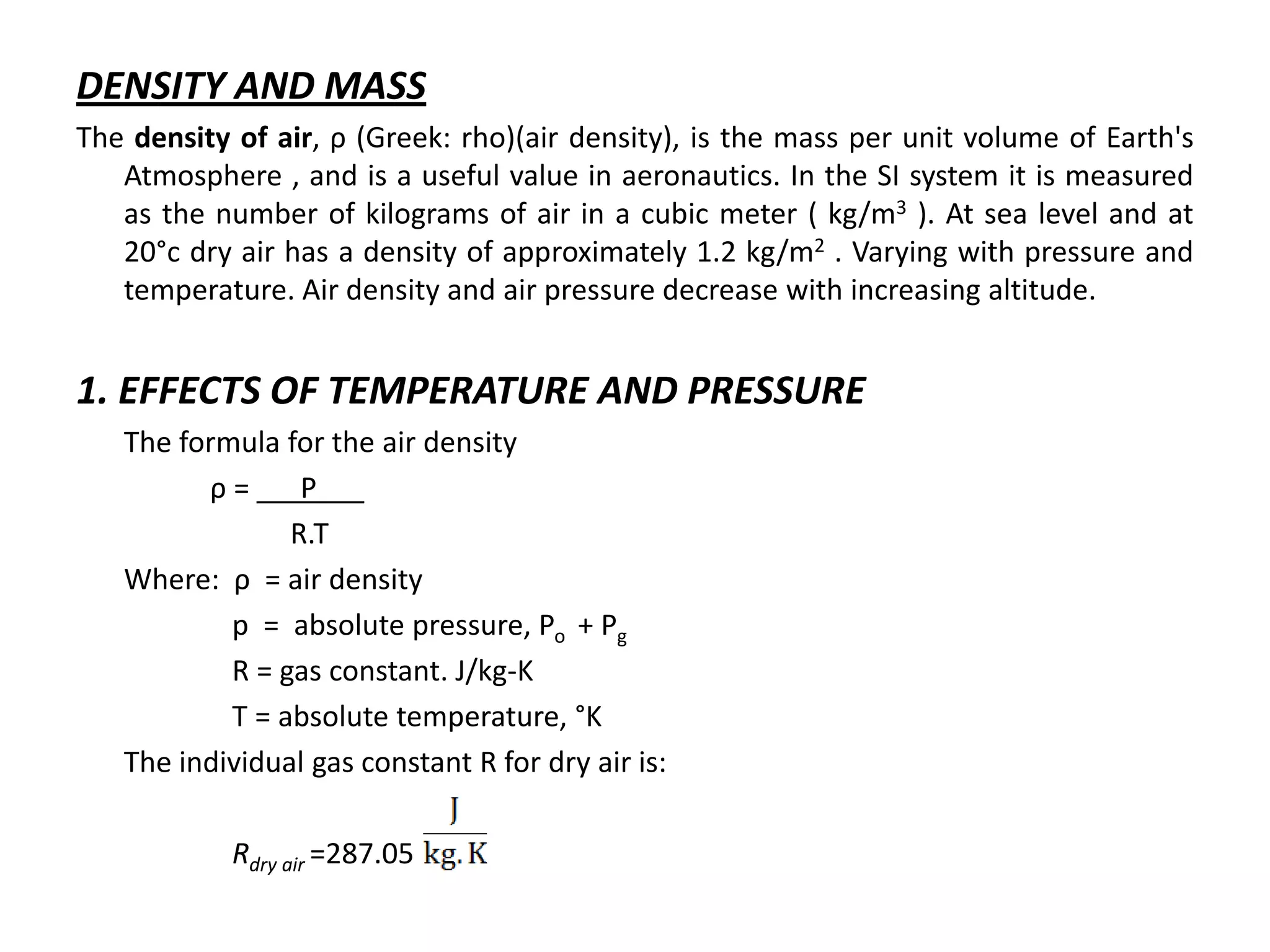
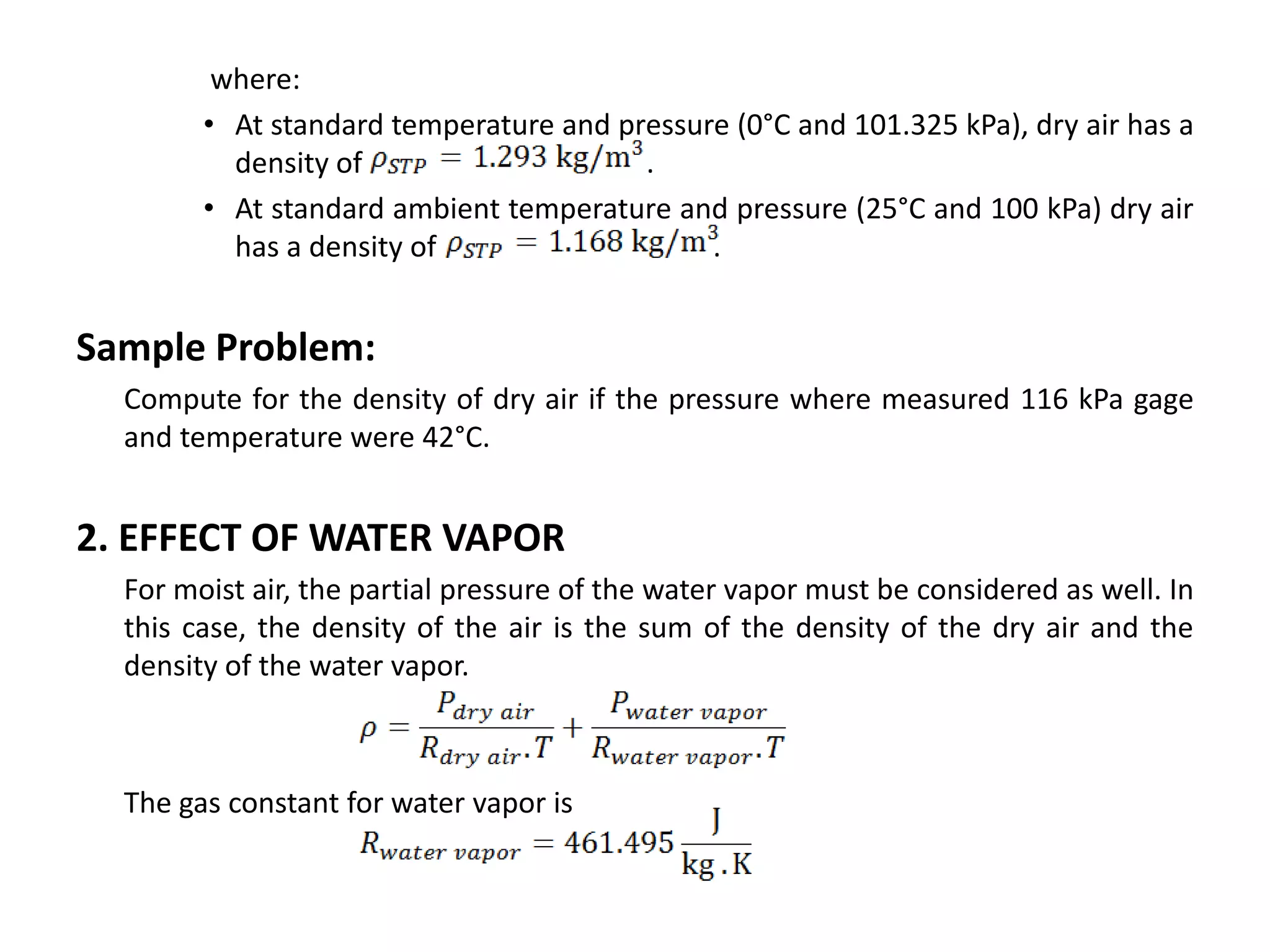
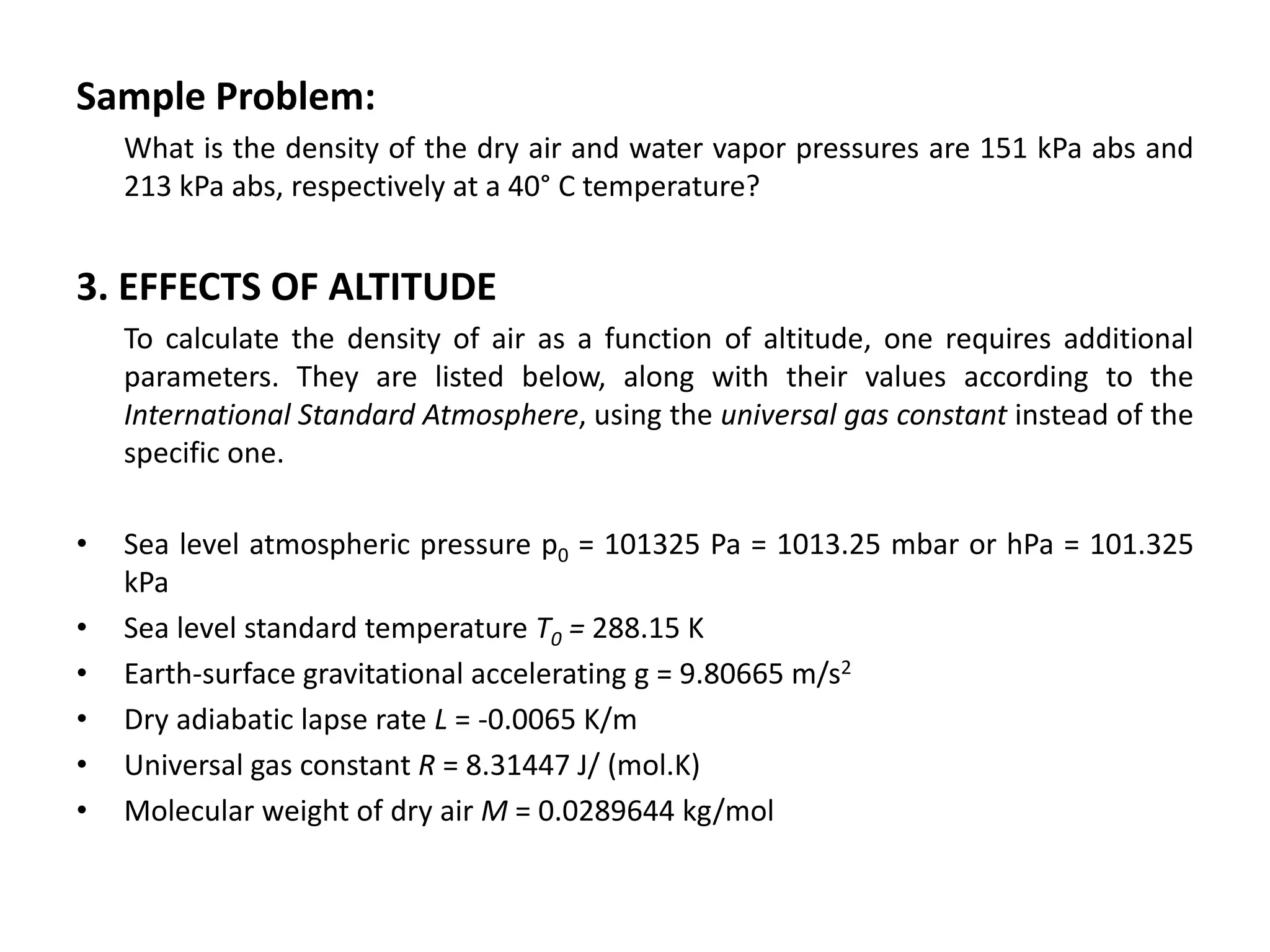
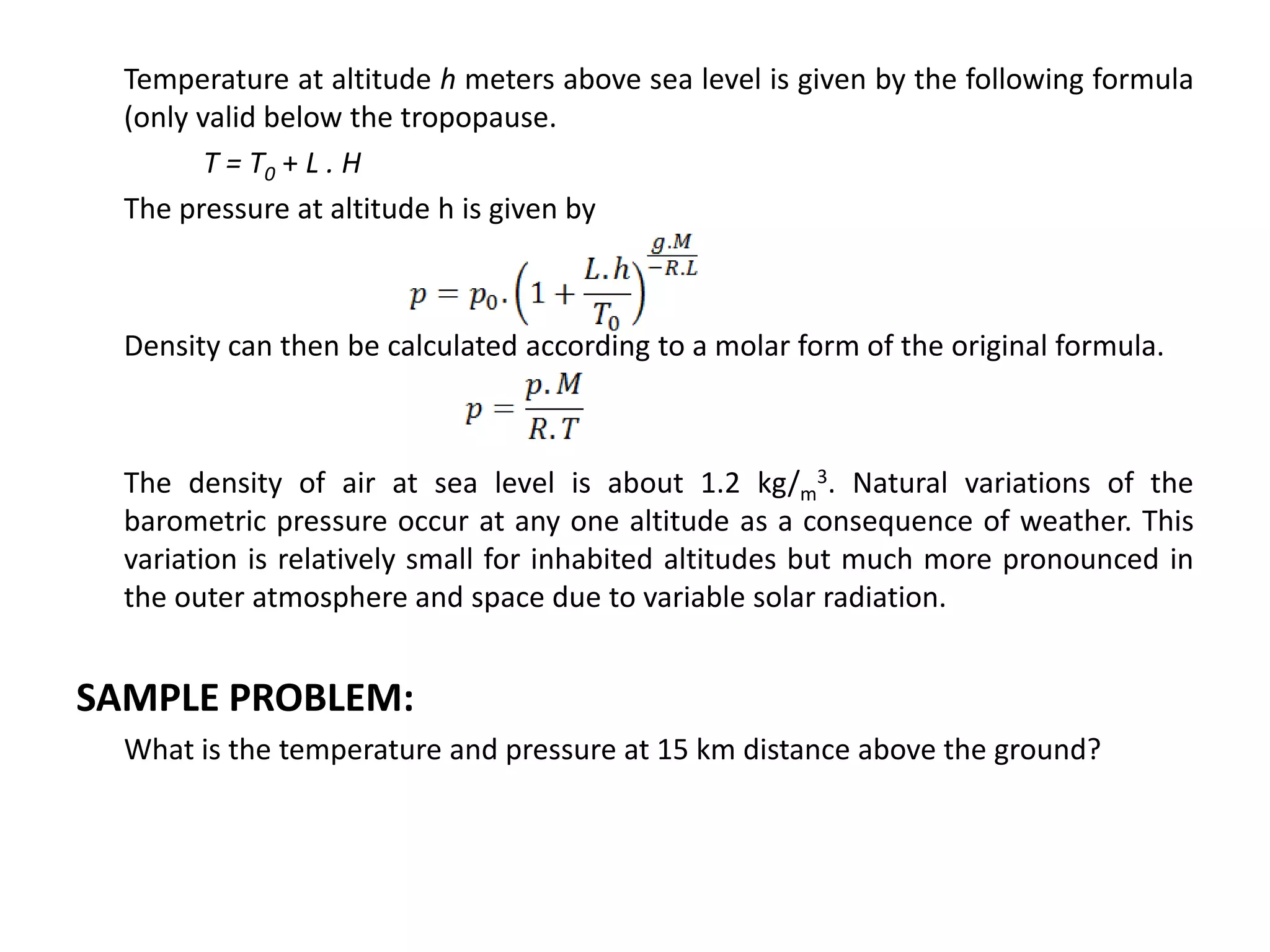
Atmospheric pressure decreases with increasing altitude due to less air above. The barometric formula models how pressure and density change with altitude, dropping off exponentially. At sea level, air has a density of about 1.2 kg/m3 under standard temperature and pressure, but density decreases with altitude as pressure and temperature drop under the effects of gravity and the dry adiabatic lapse rate.