The document provides information about physics concepts related to pressure and temperature measurement. It discusses:
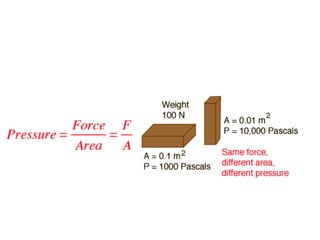
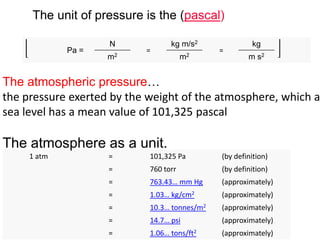
- Definitions of pressure, standard pressure units like Pascal and atmospheric pressure in different units.
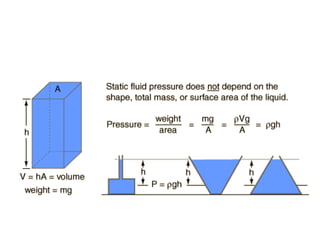
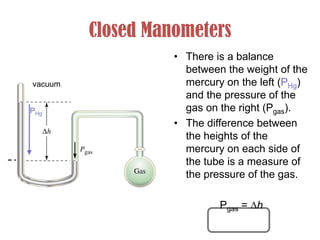
- Relationship between pressure, density, height of liquid and gravity.
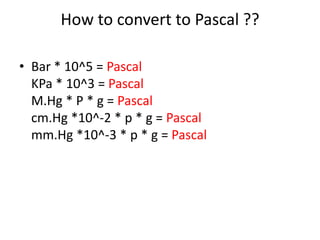
- Conversion between different pressure units.
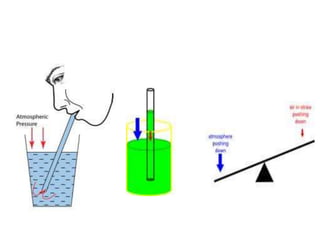
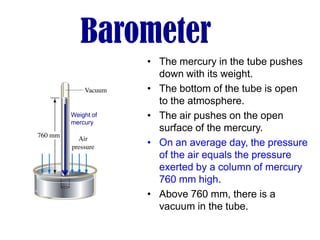
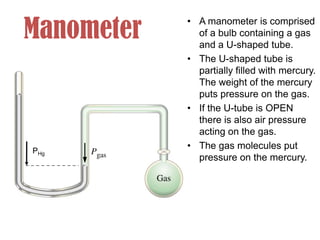
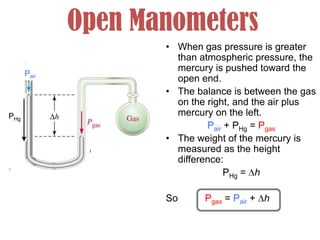
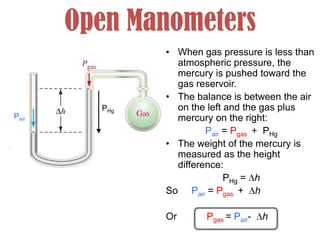
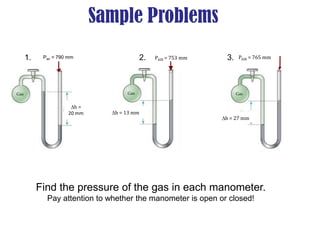
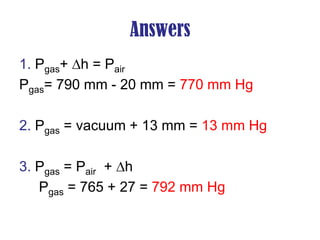
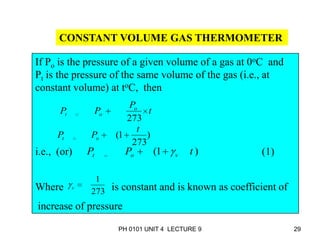
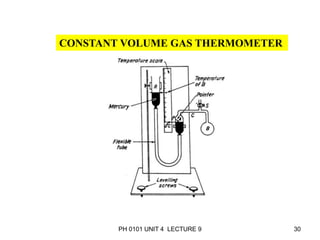
- Operation of barometers, manometers and gas thermometers to measure pressure and temperature. Barometers use mercury columns, while manometers use liquid columns differentially to measure gas pressures. Gas thermometers use the direct relationship between gas pressure and temperature at constant volume.