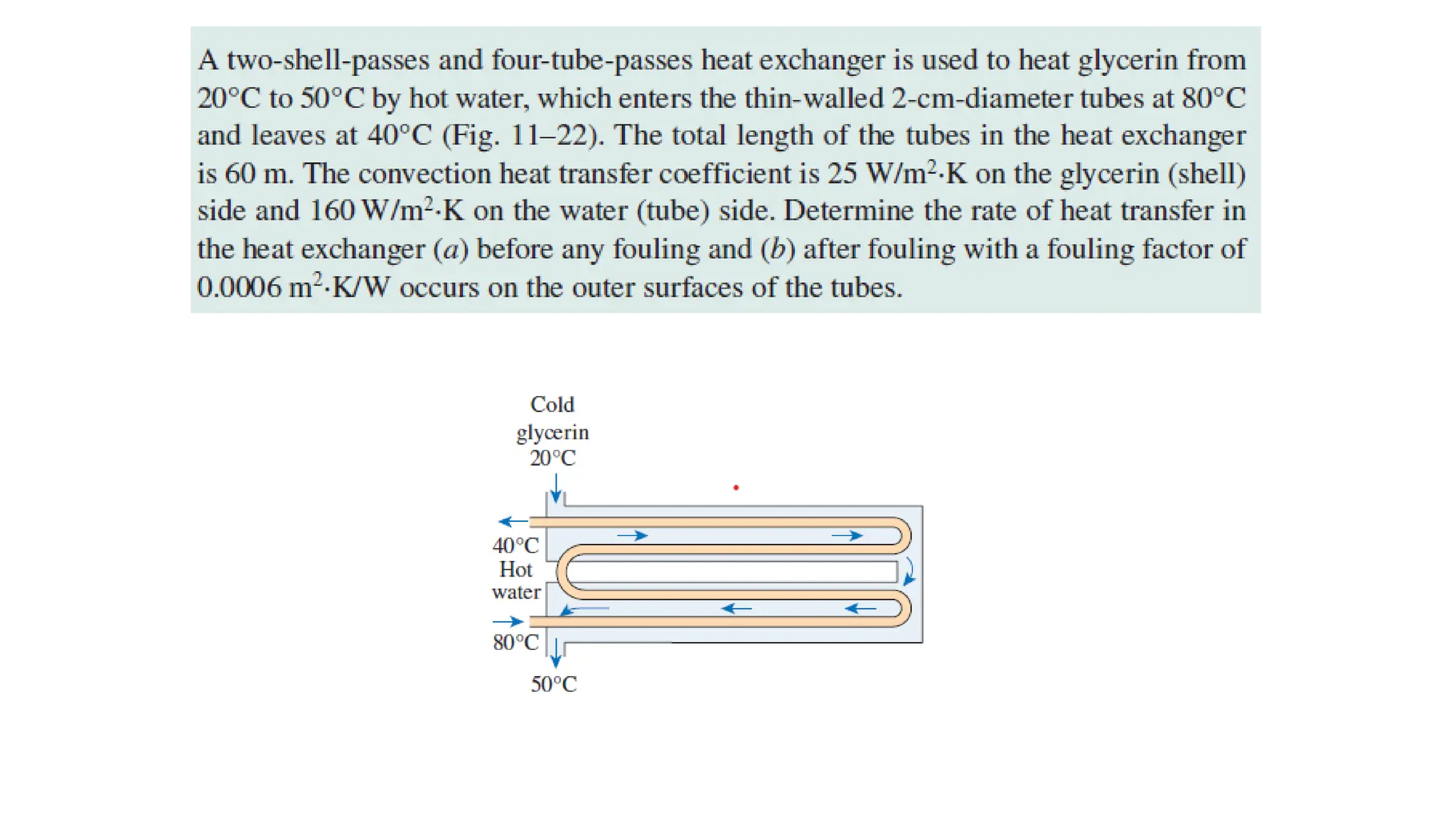
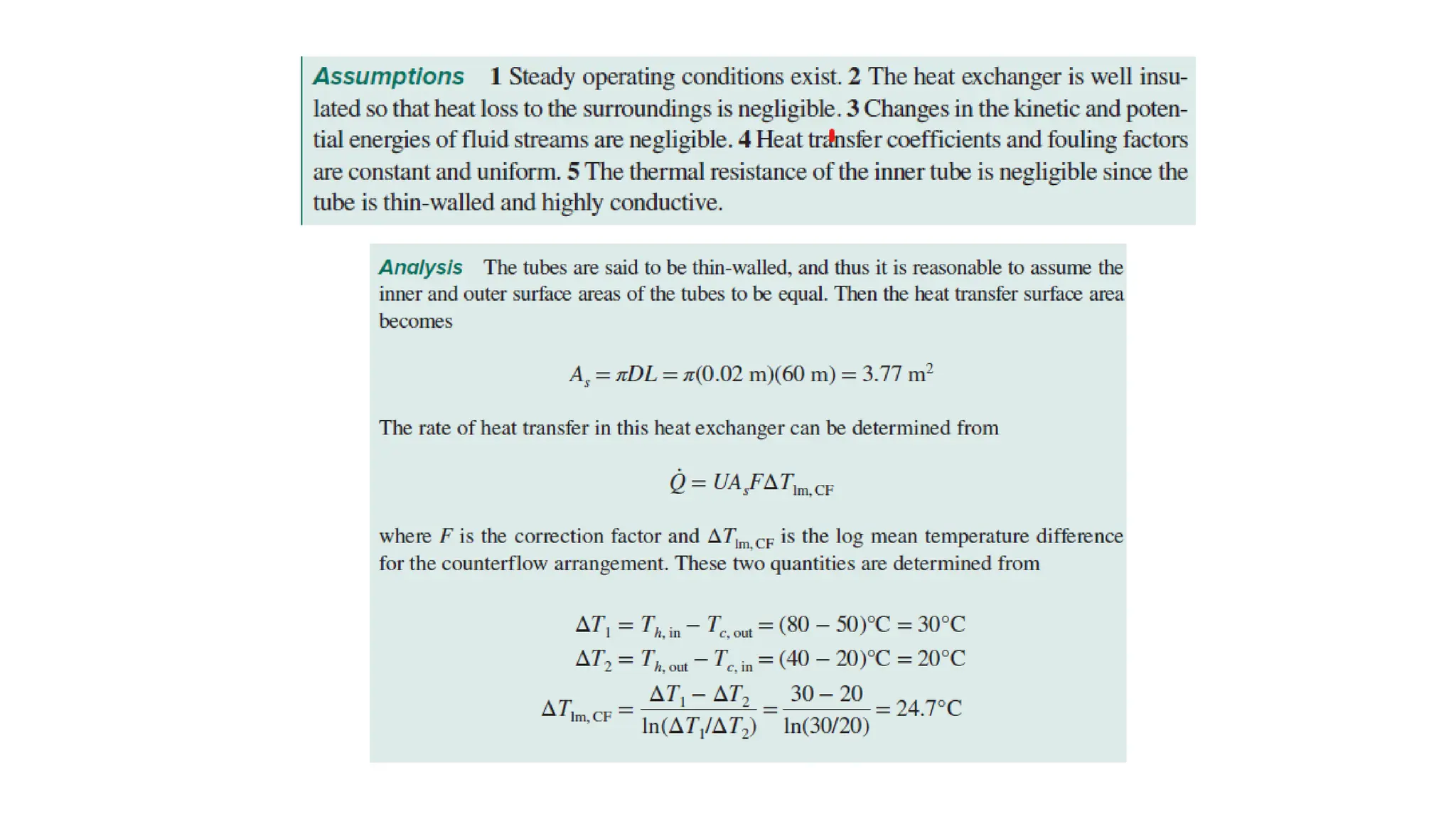
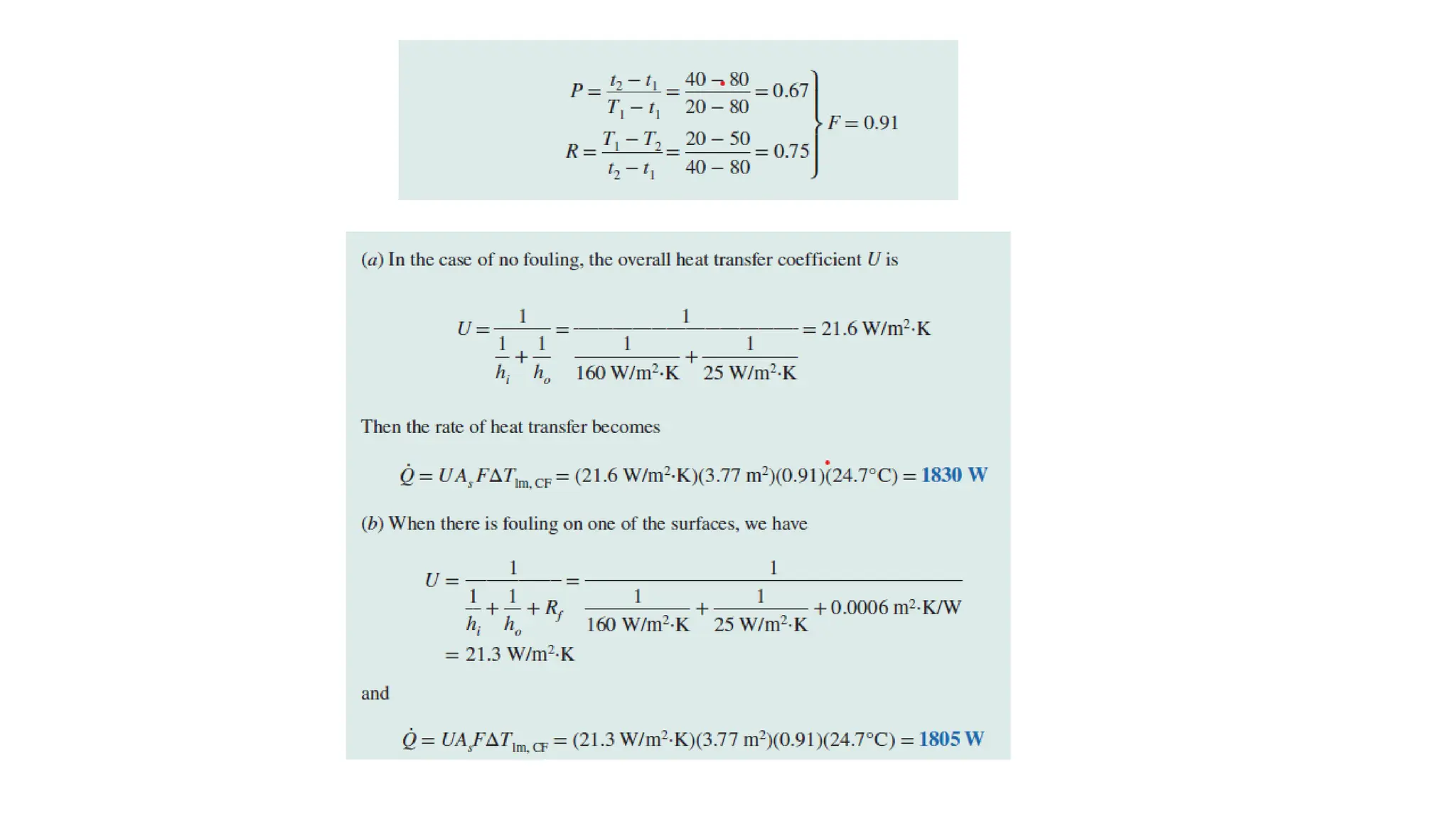
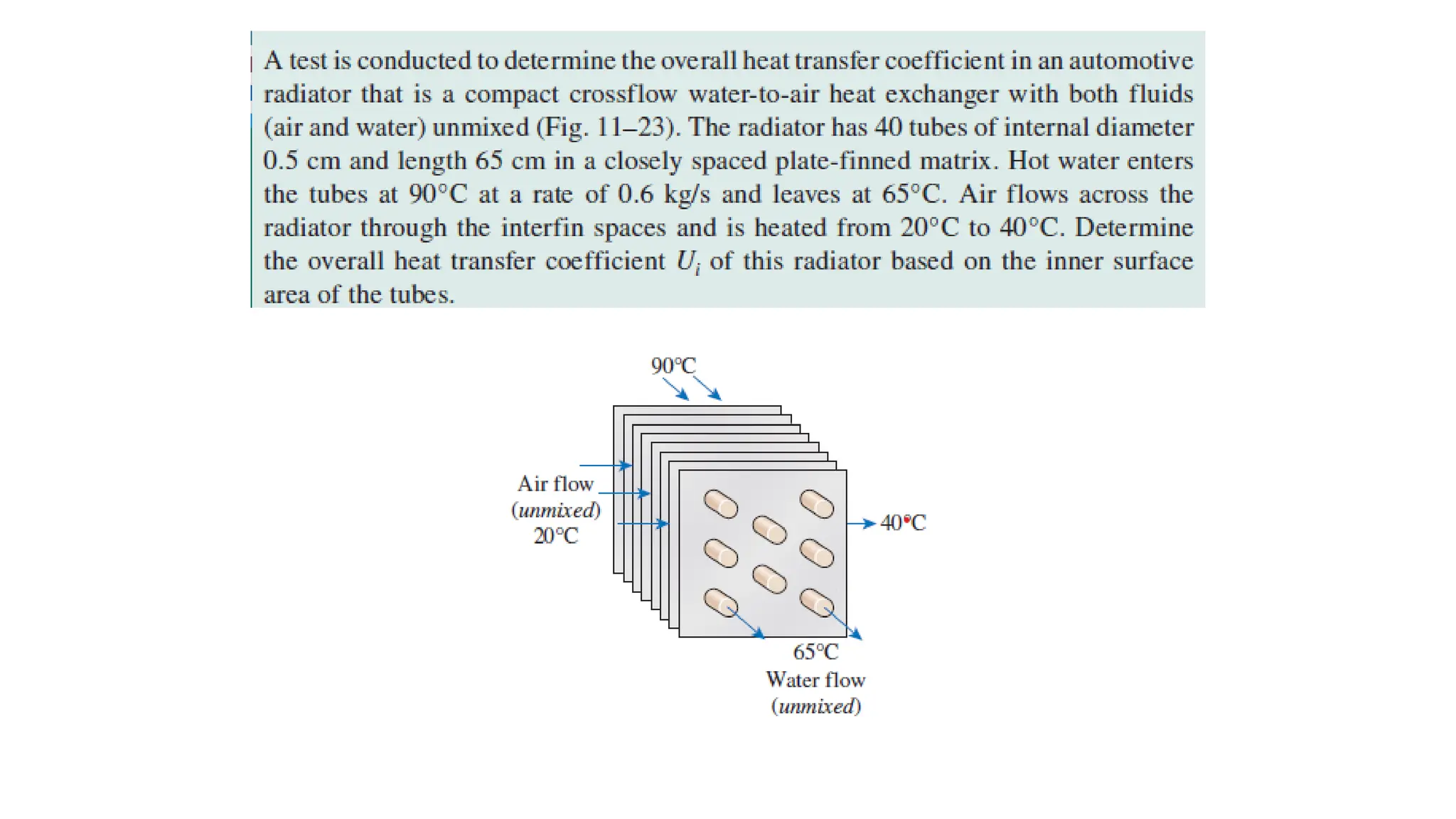
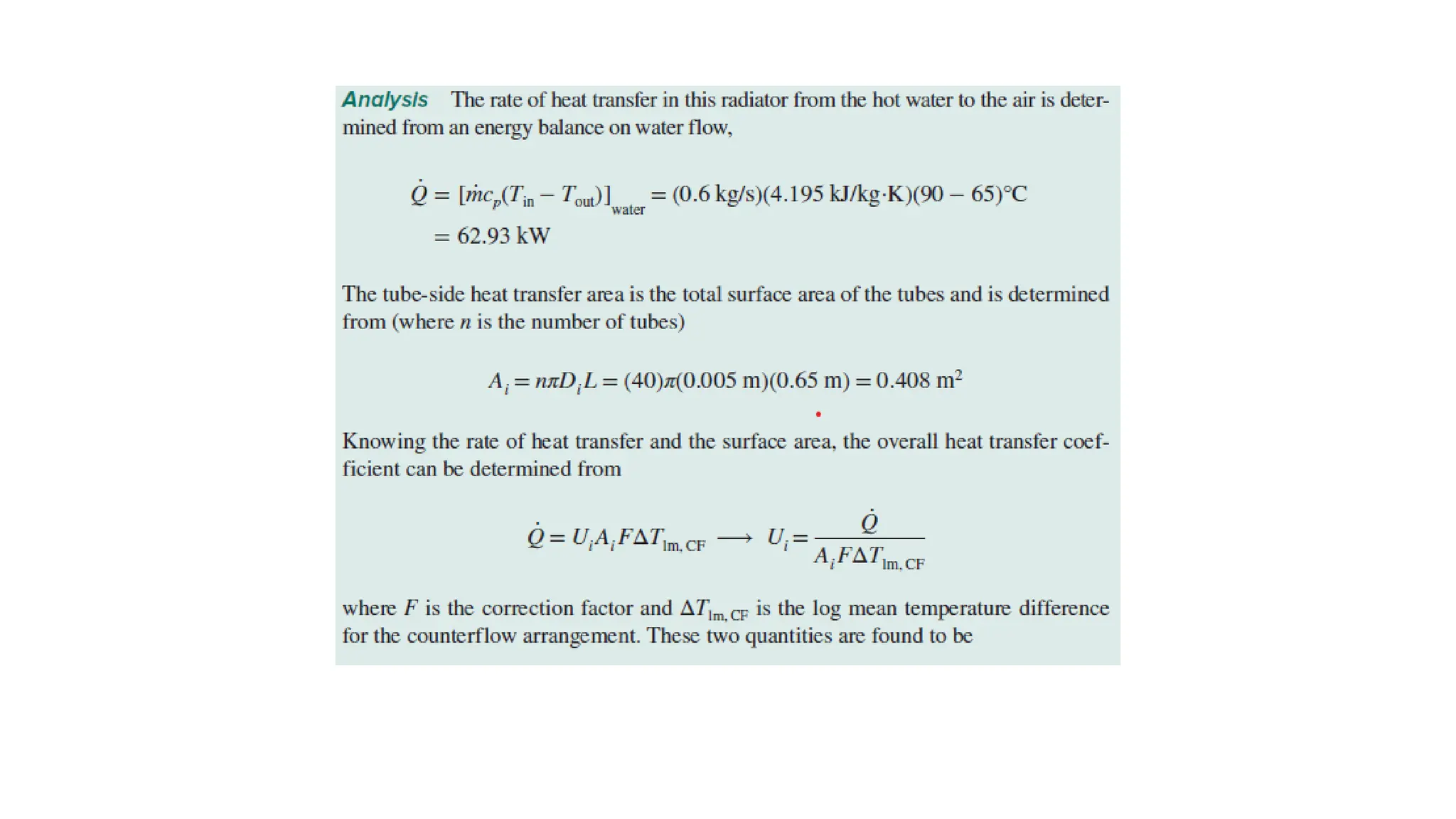
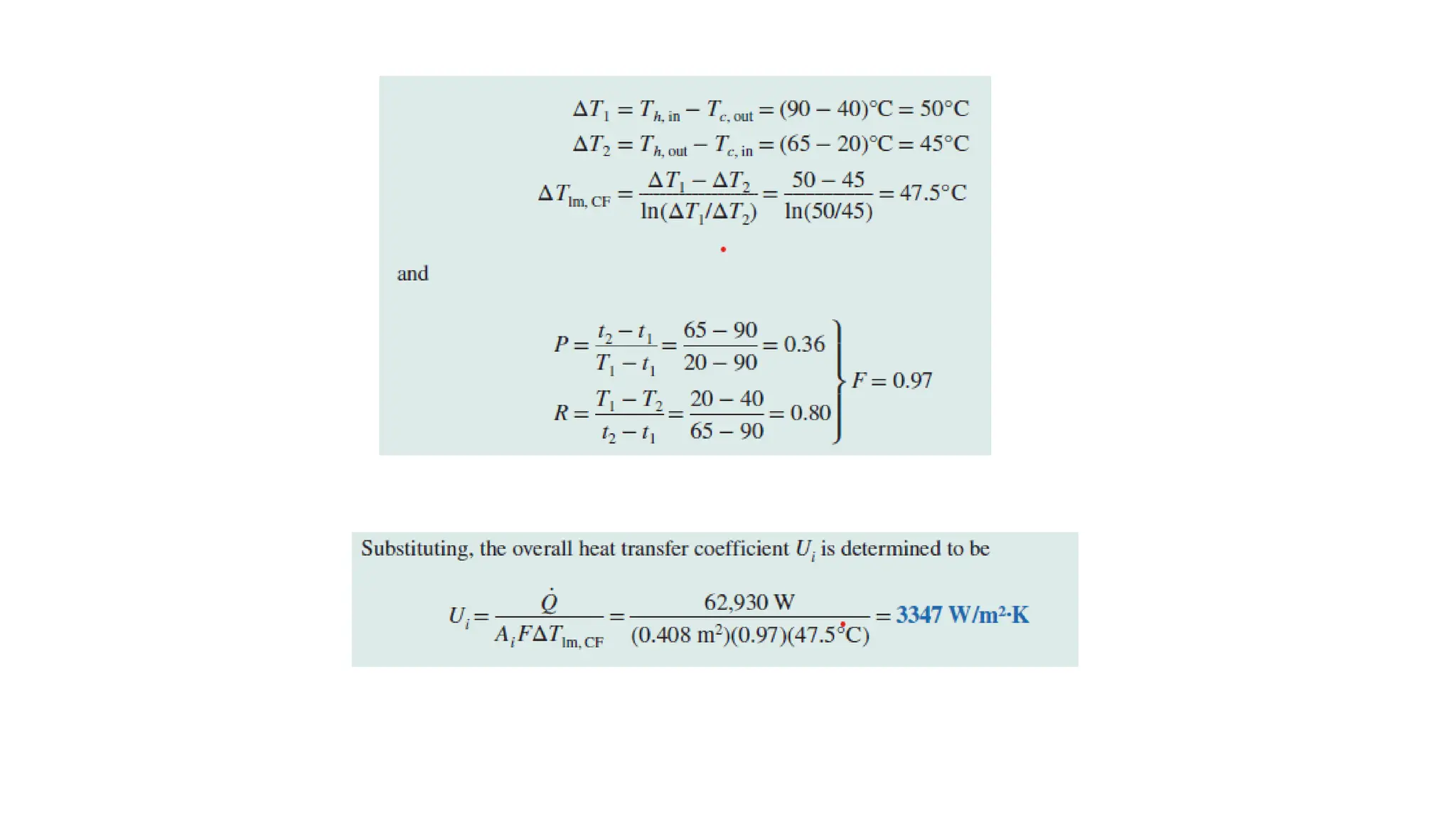
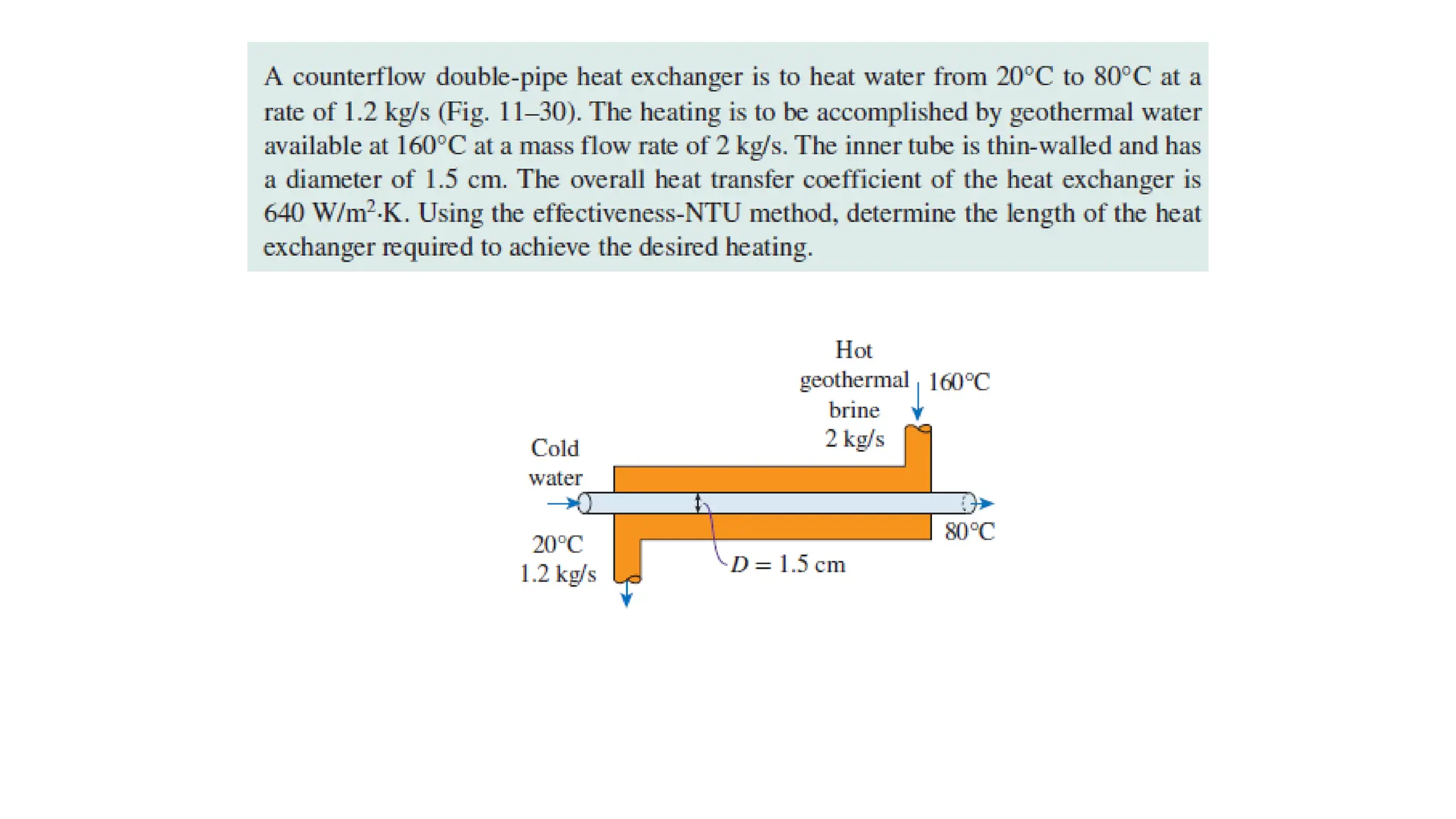
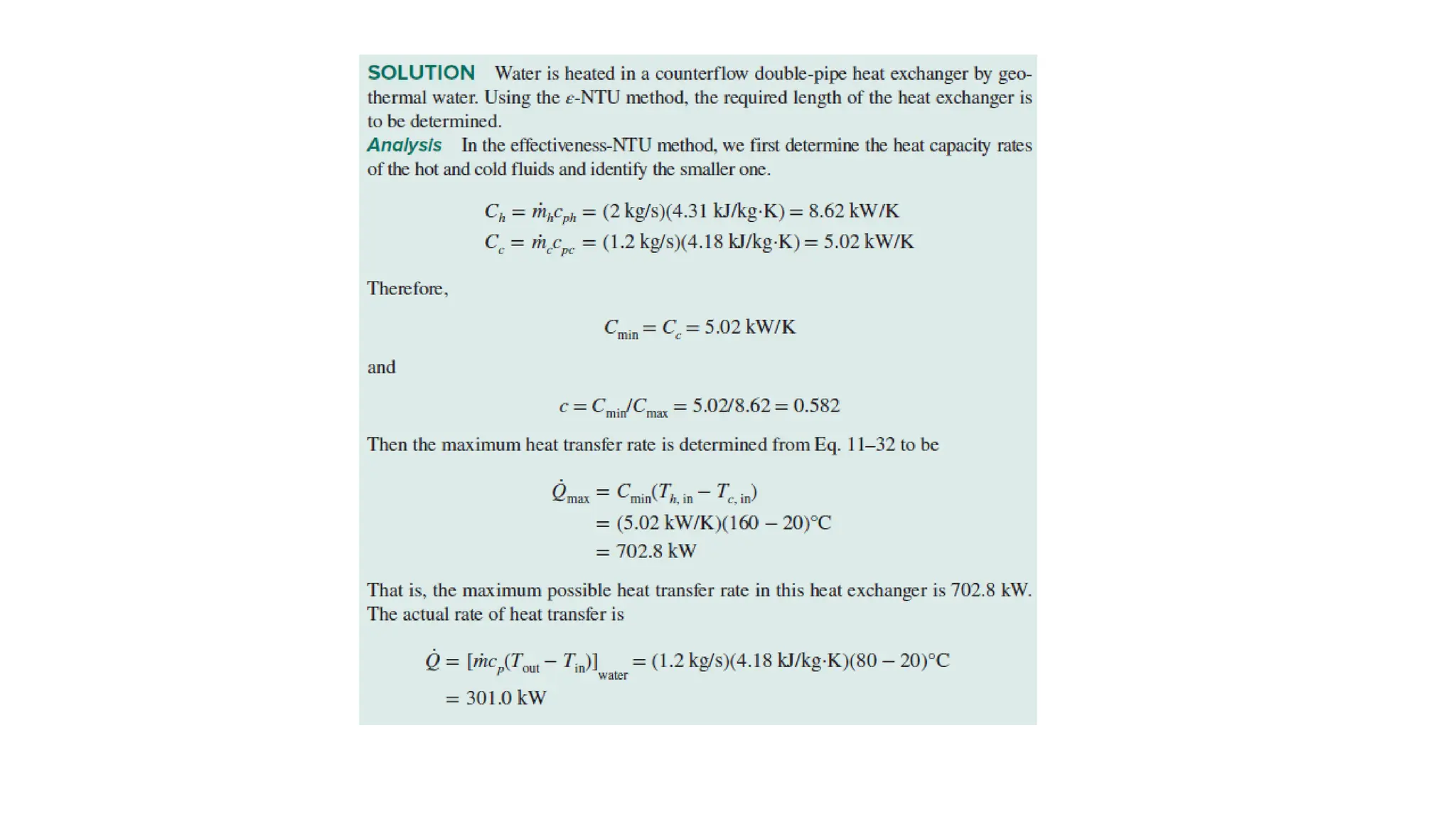
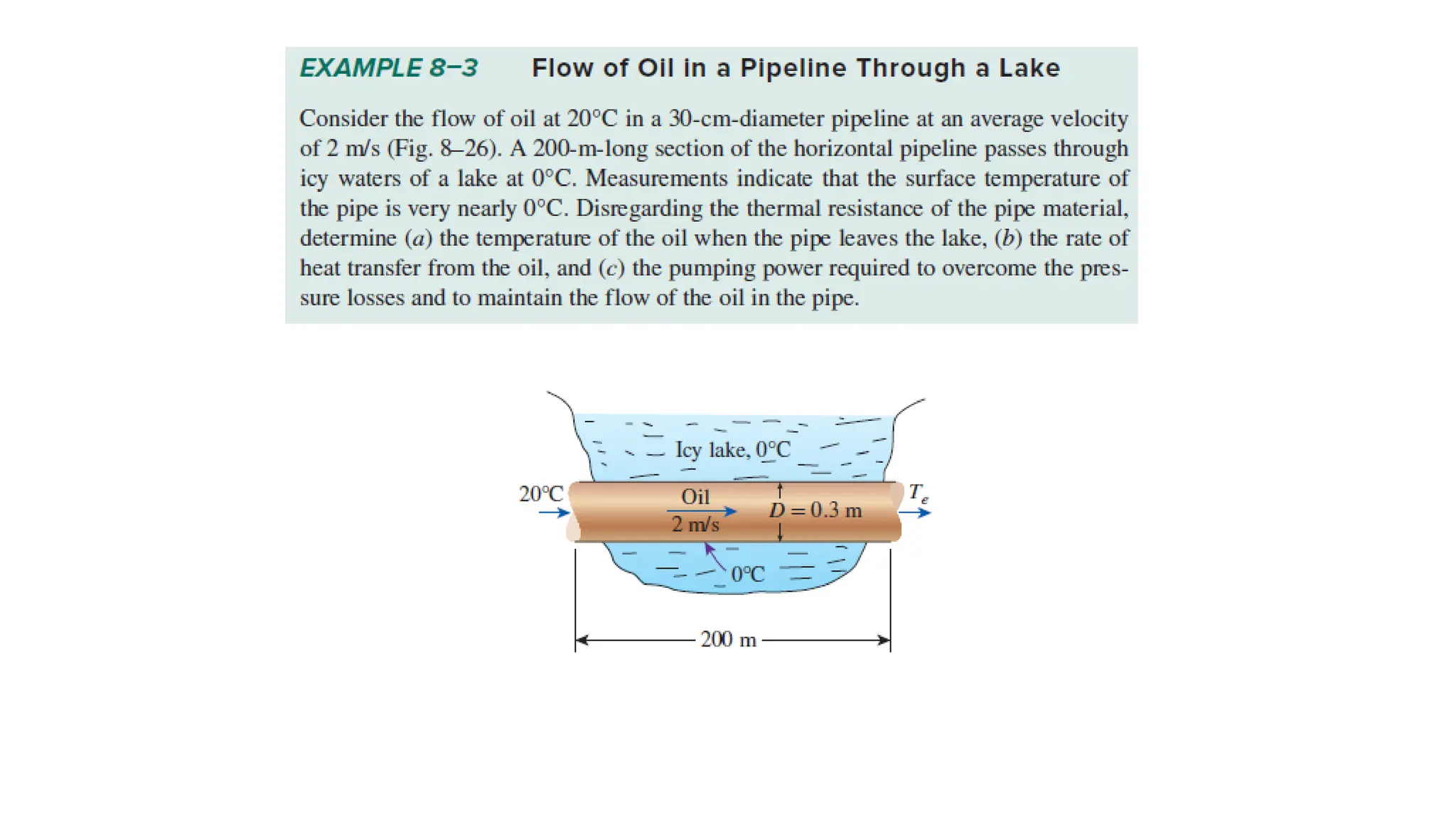
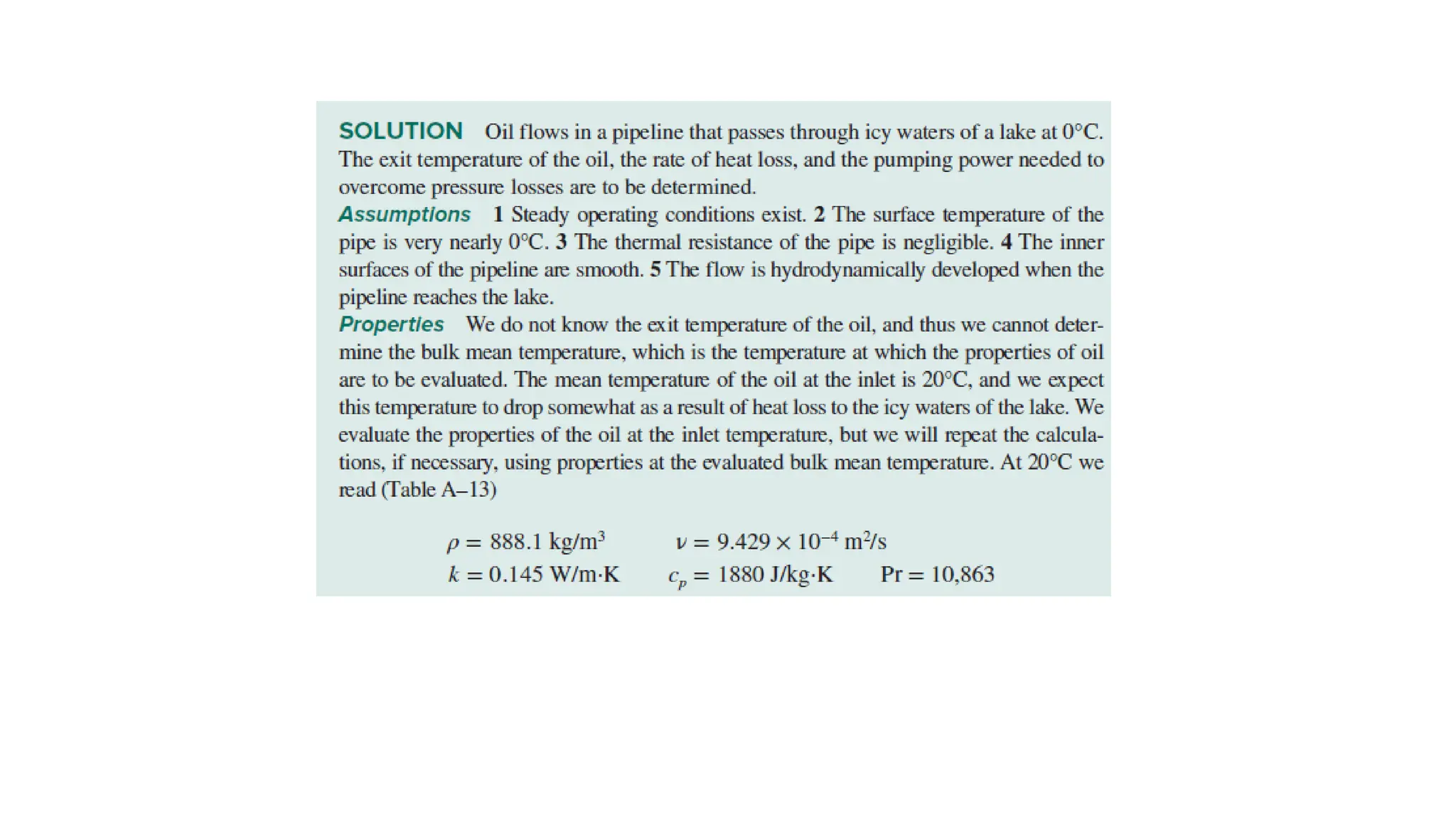
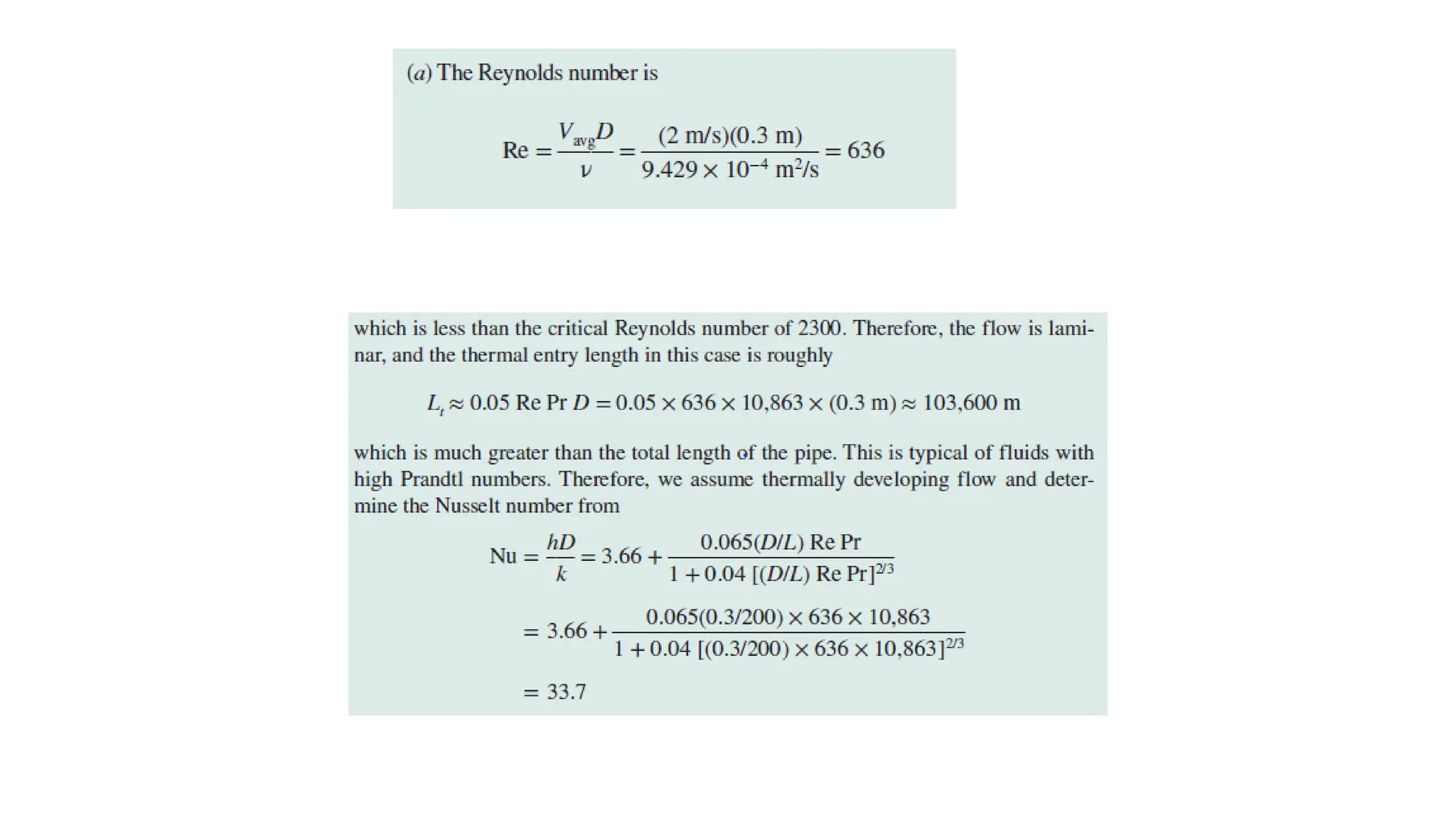
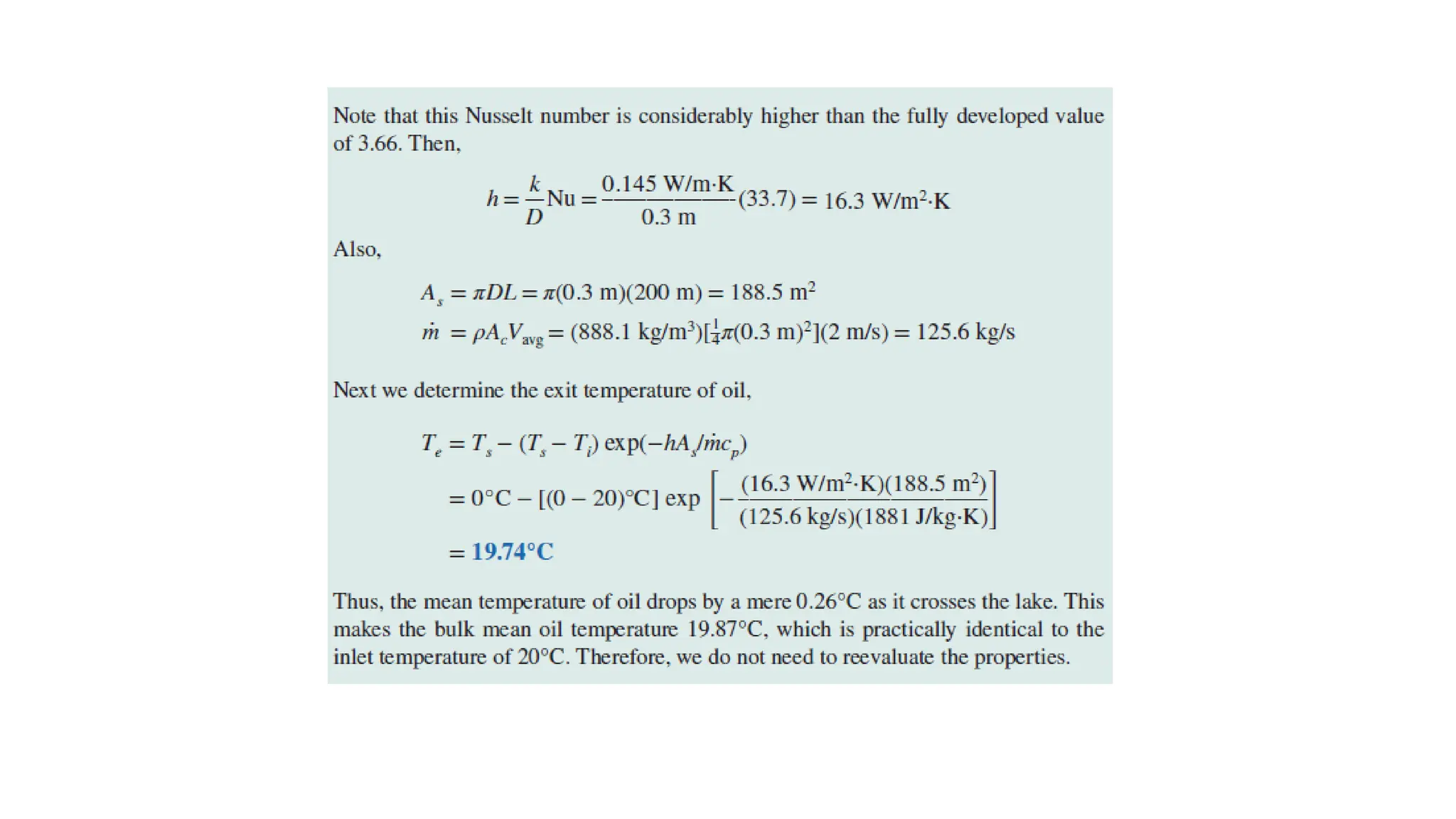
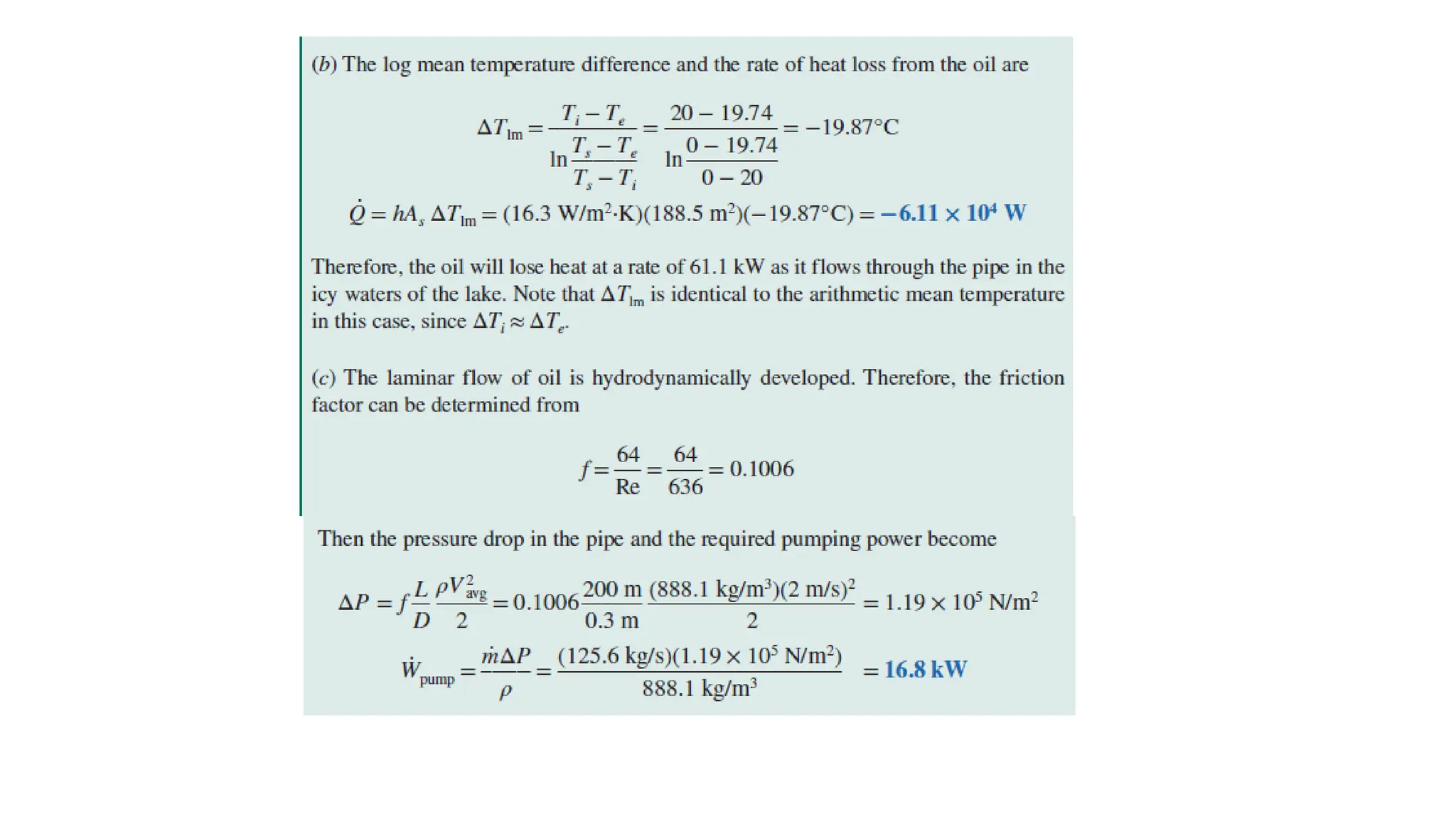
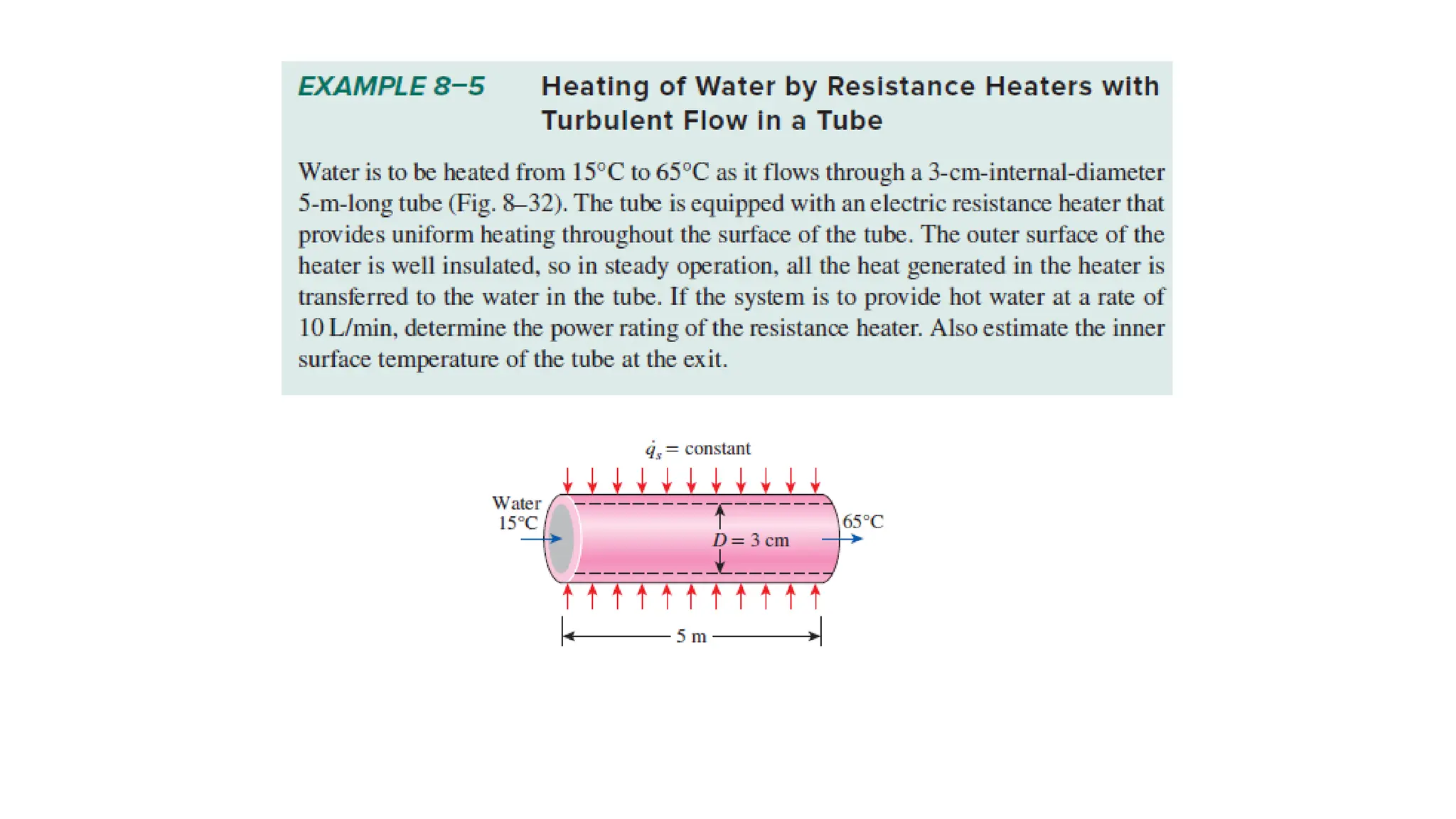
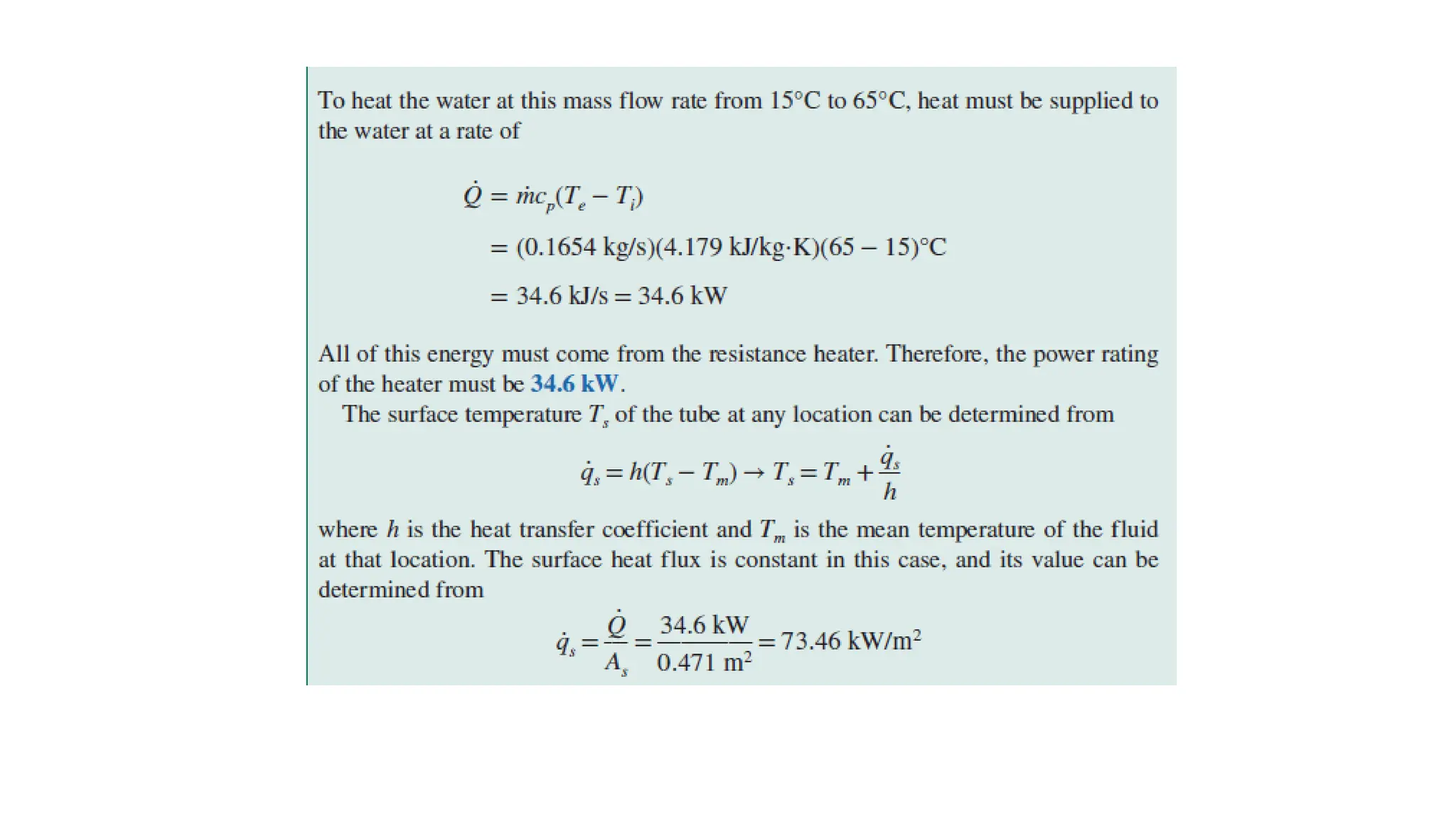
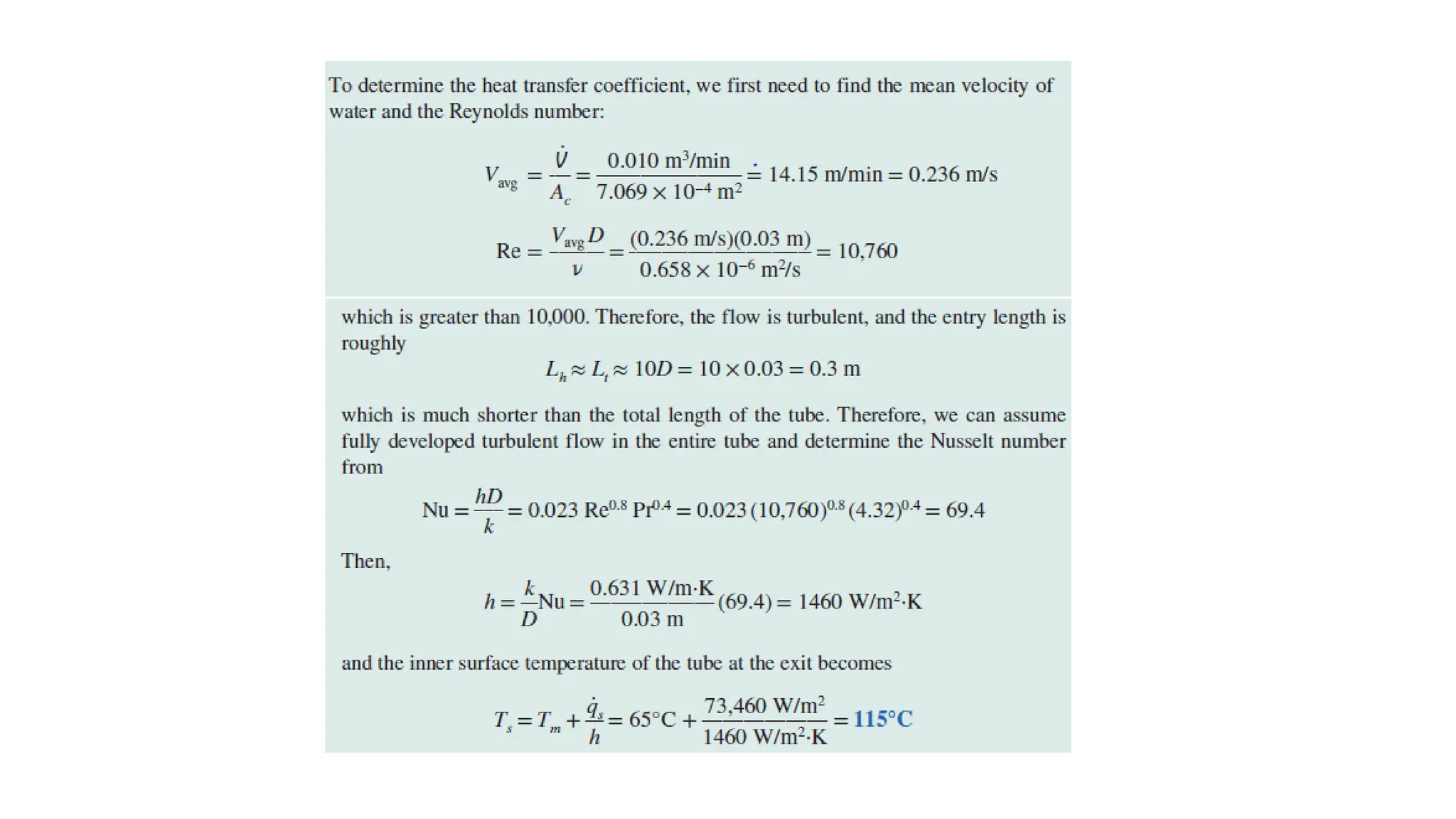
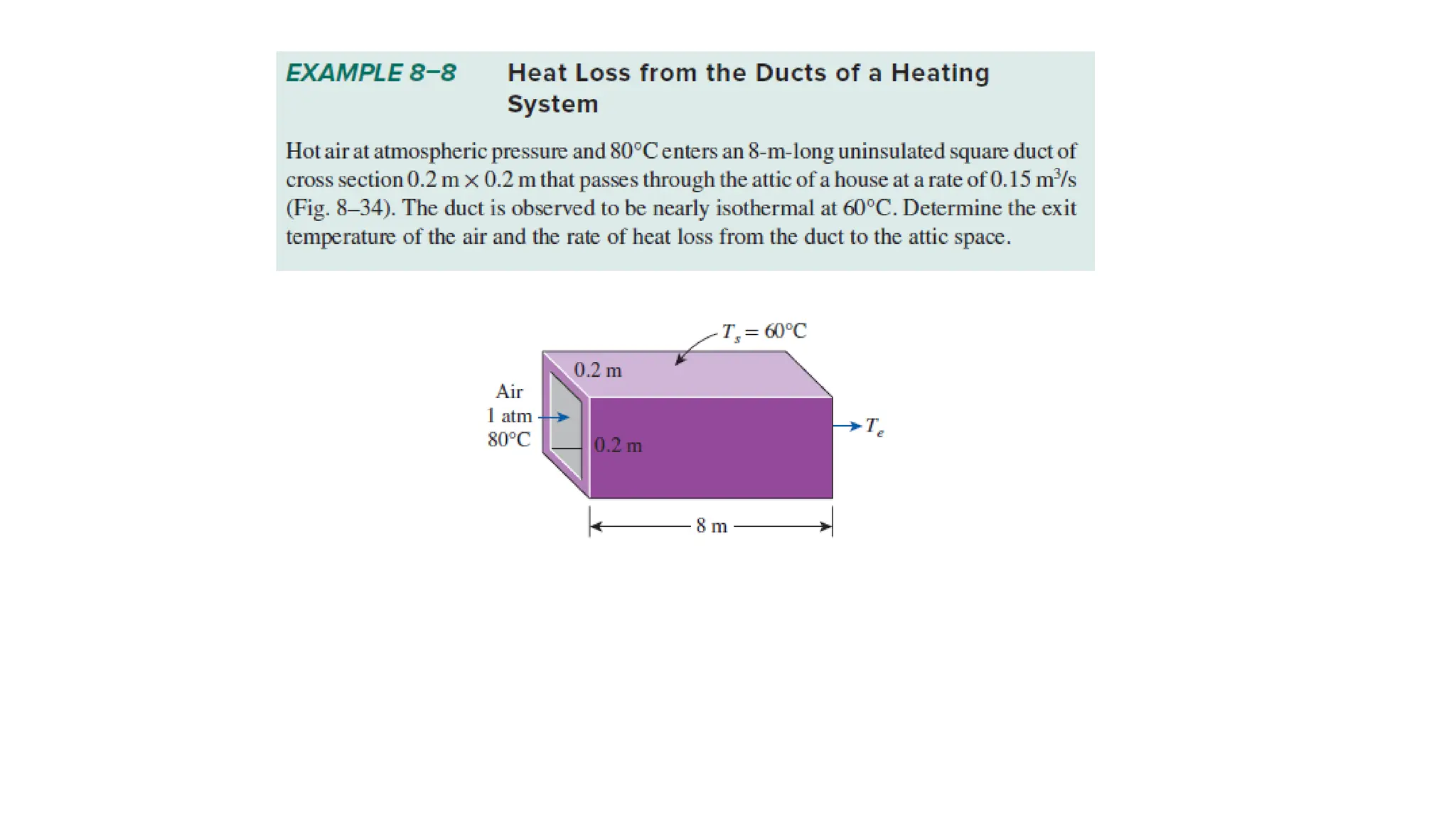
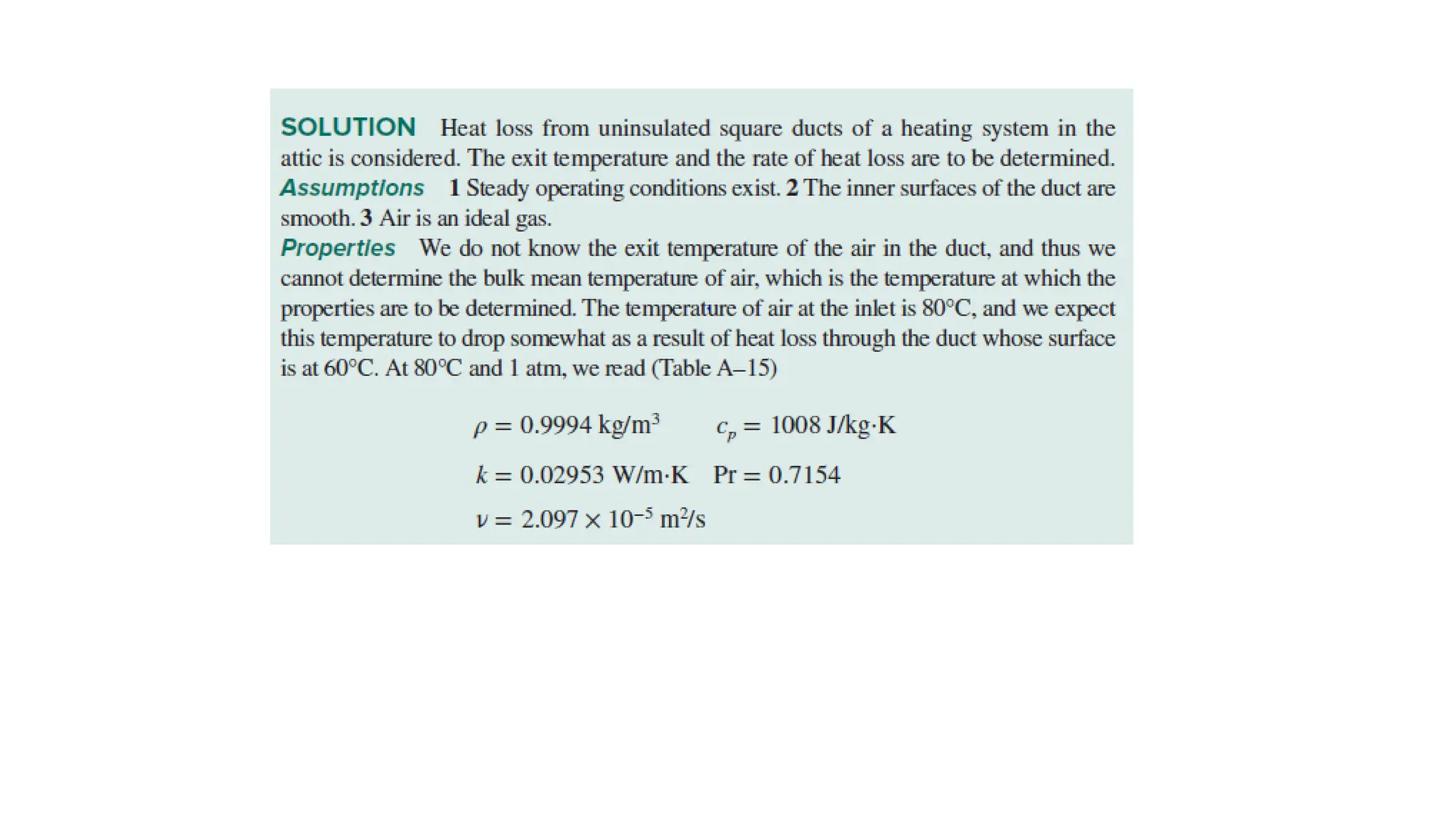
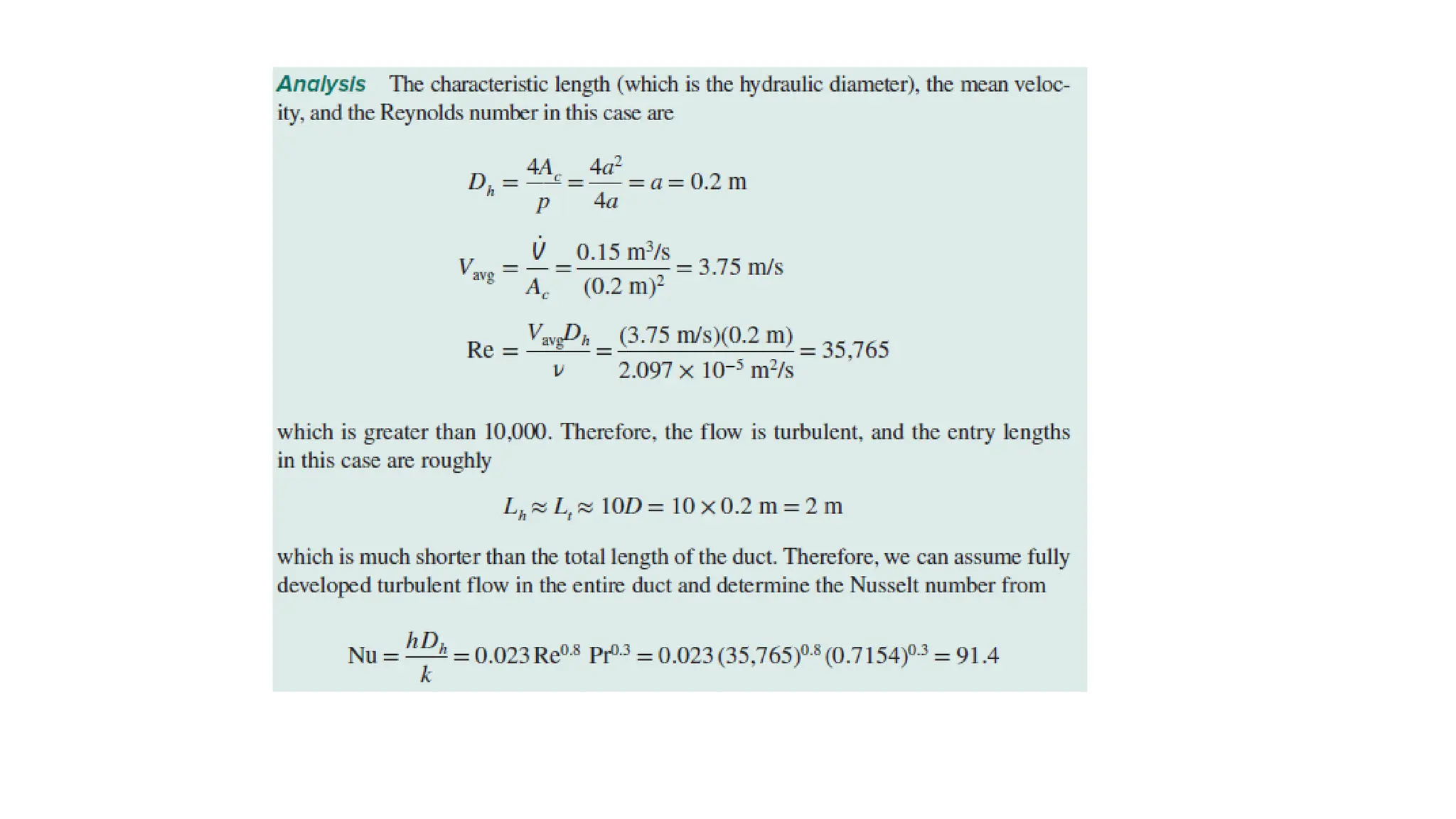
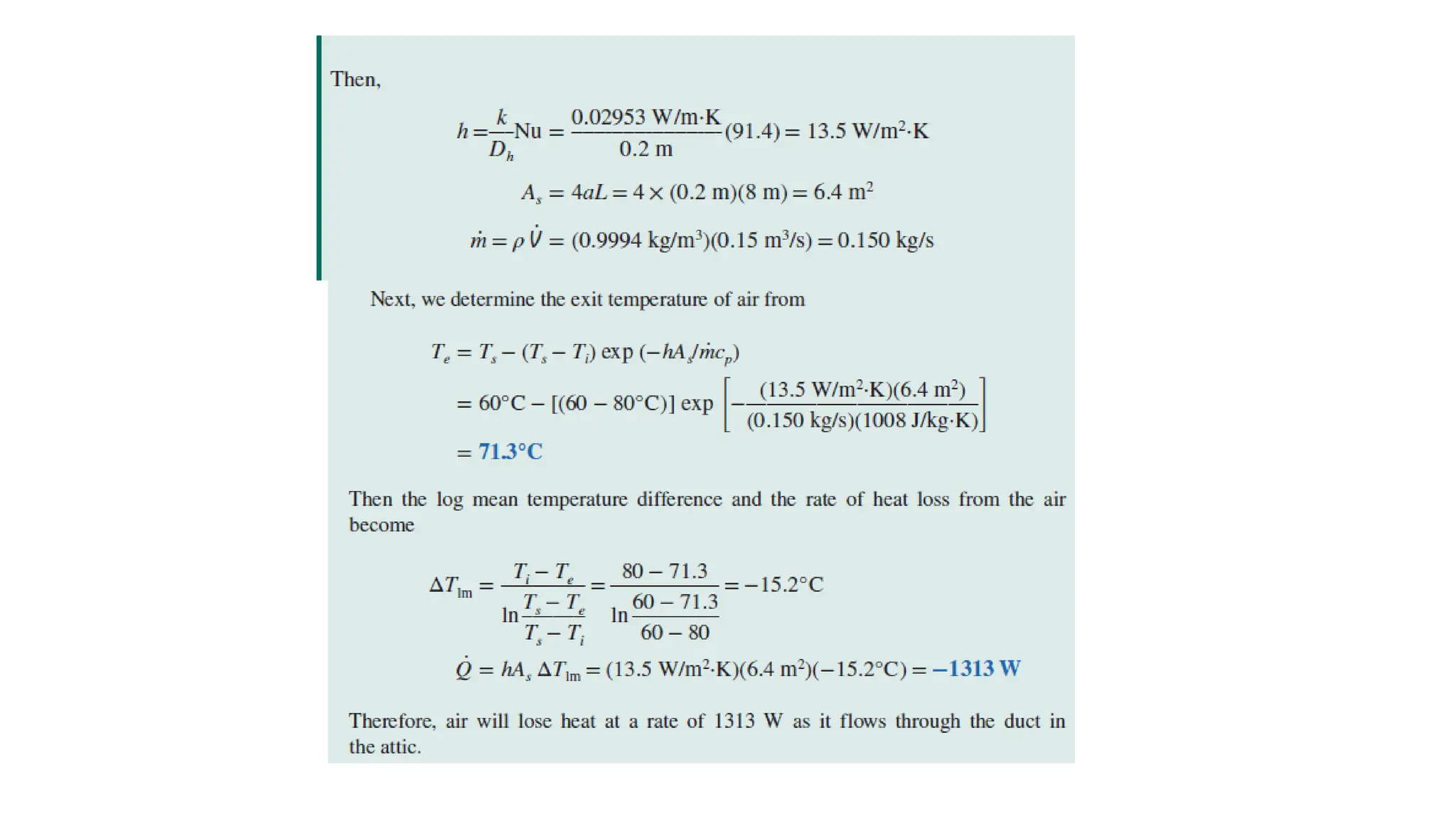
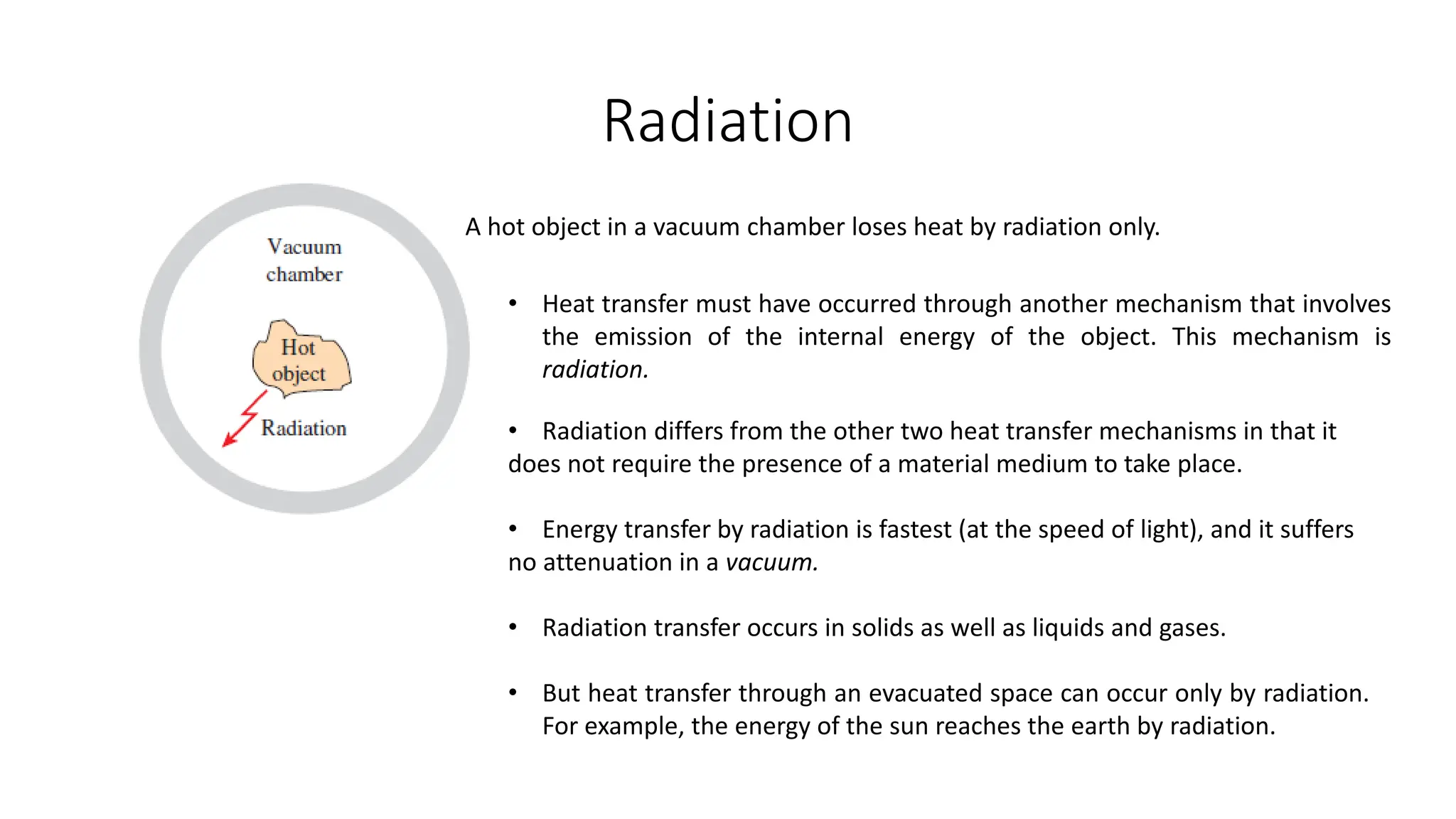
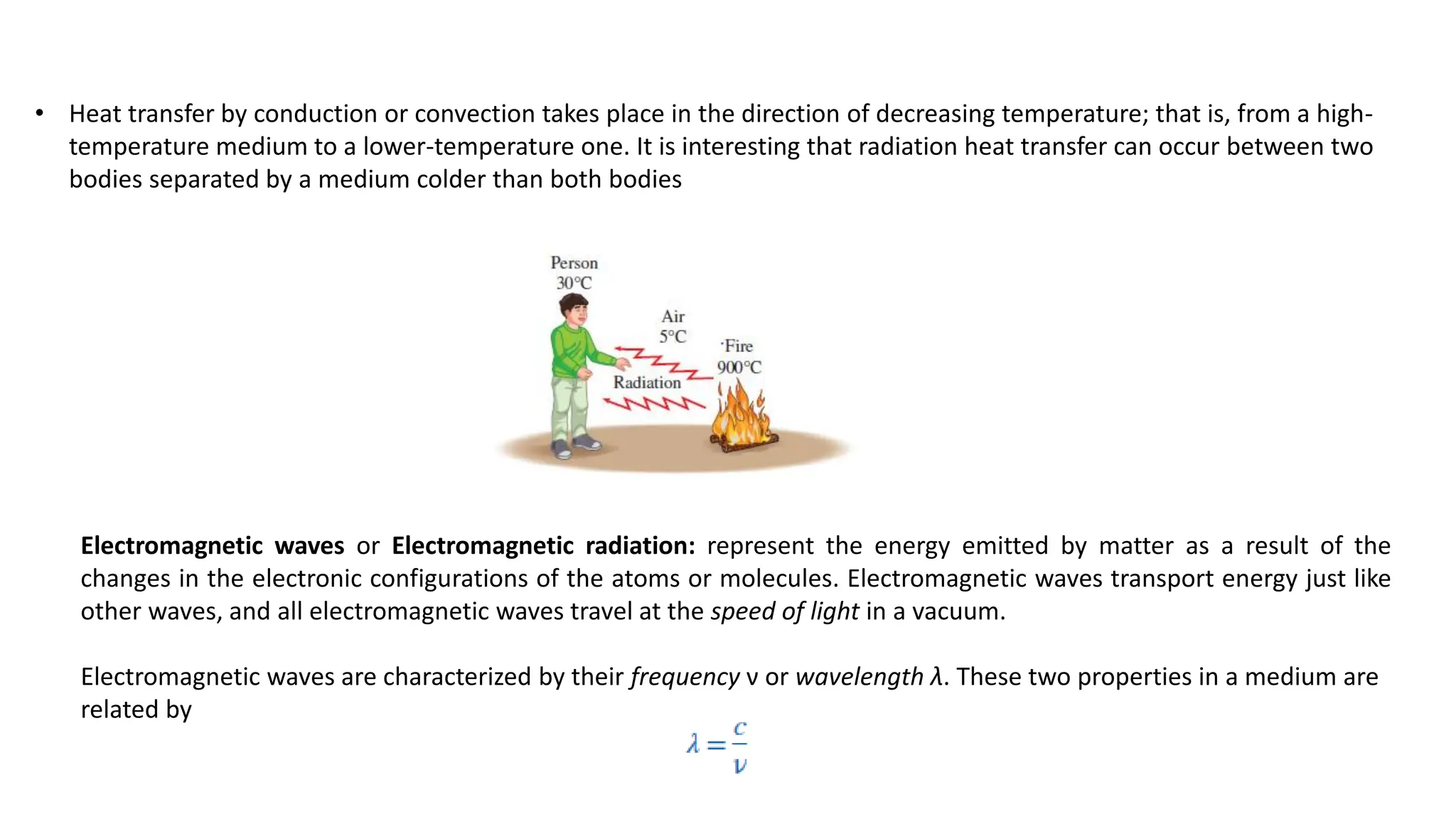
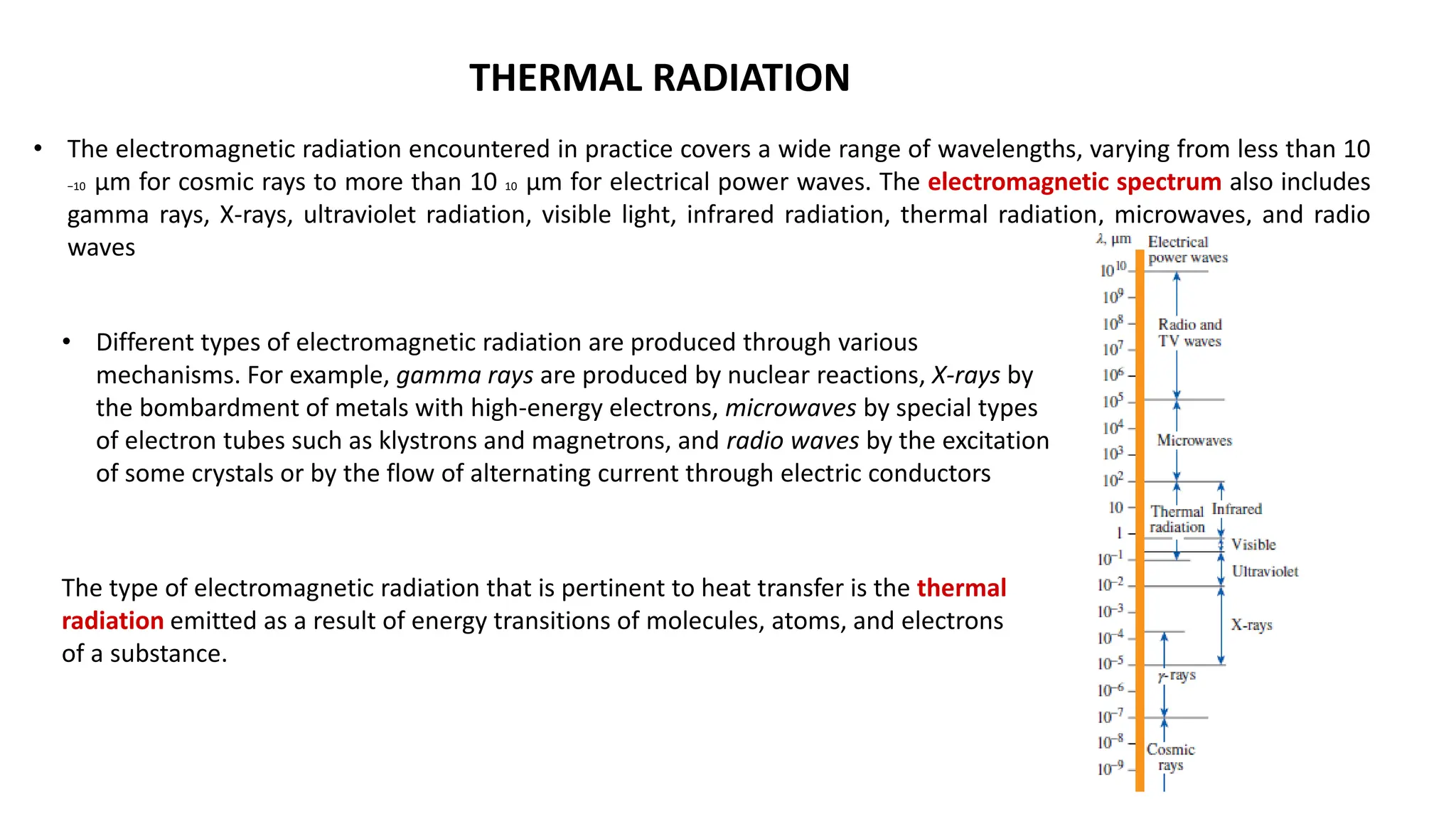
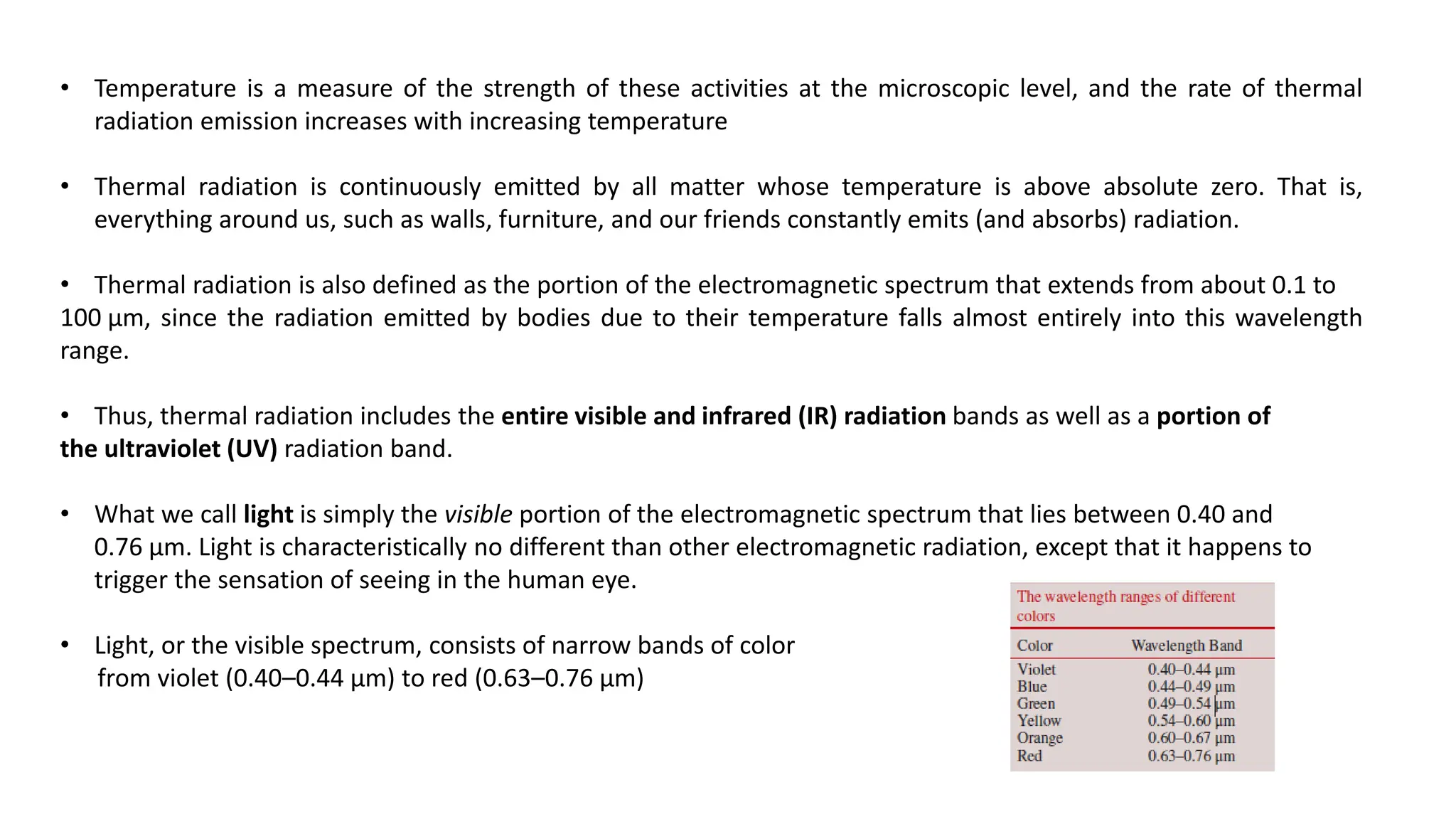
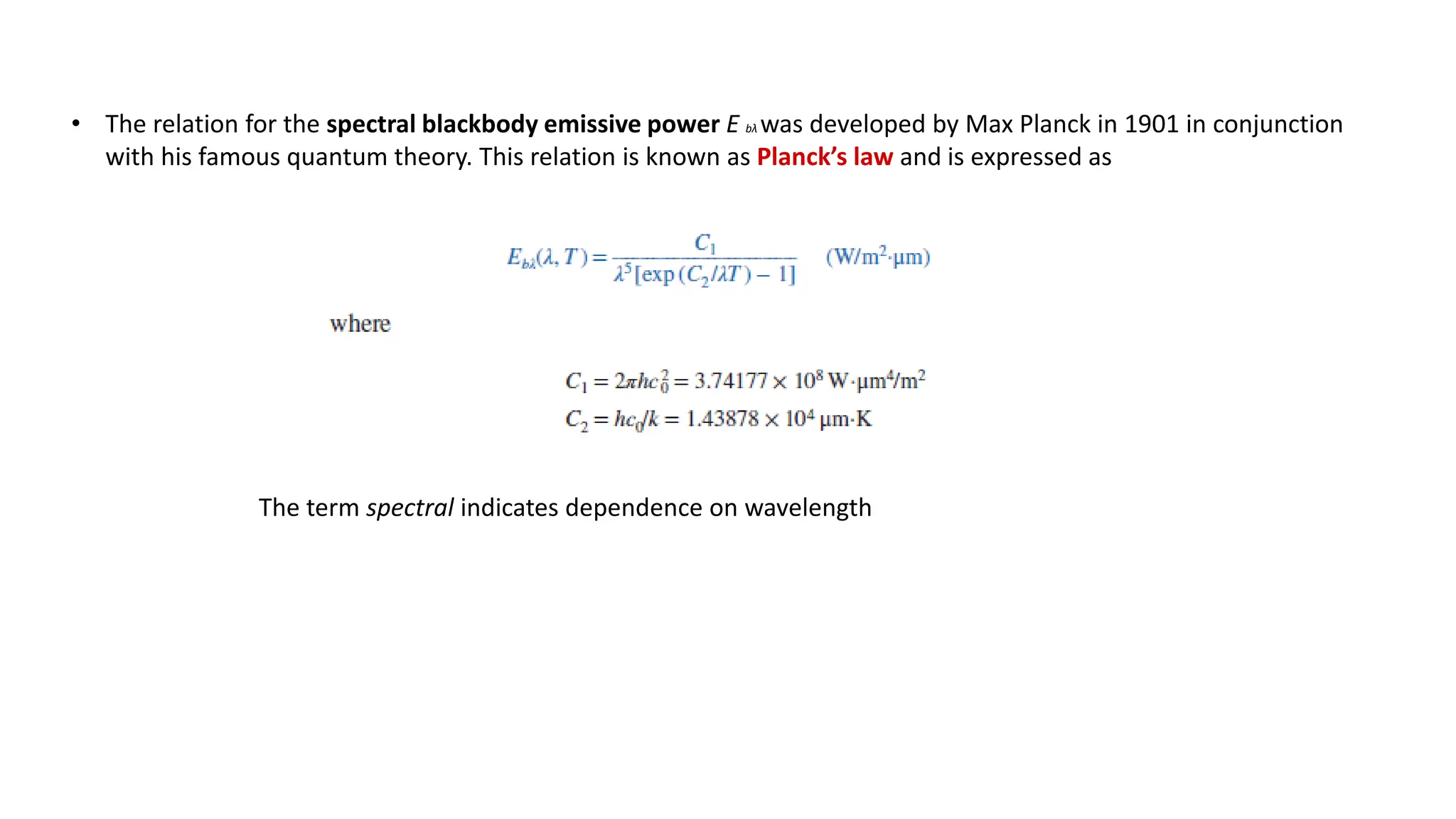
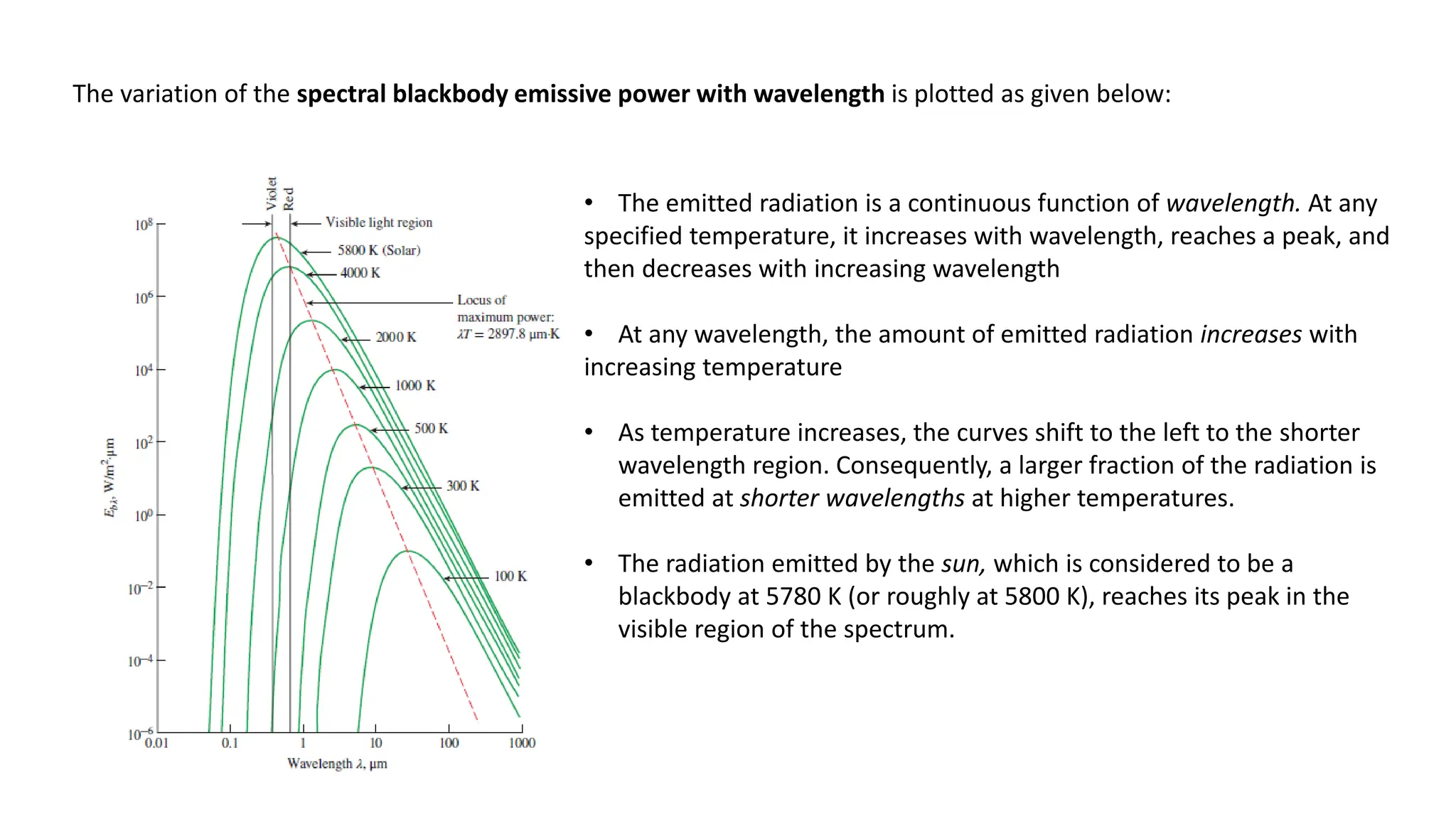
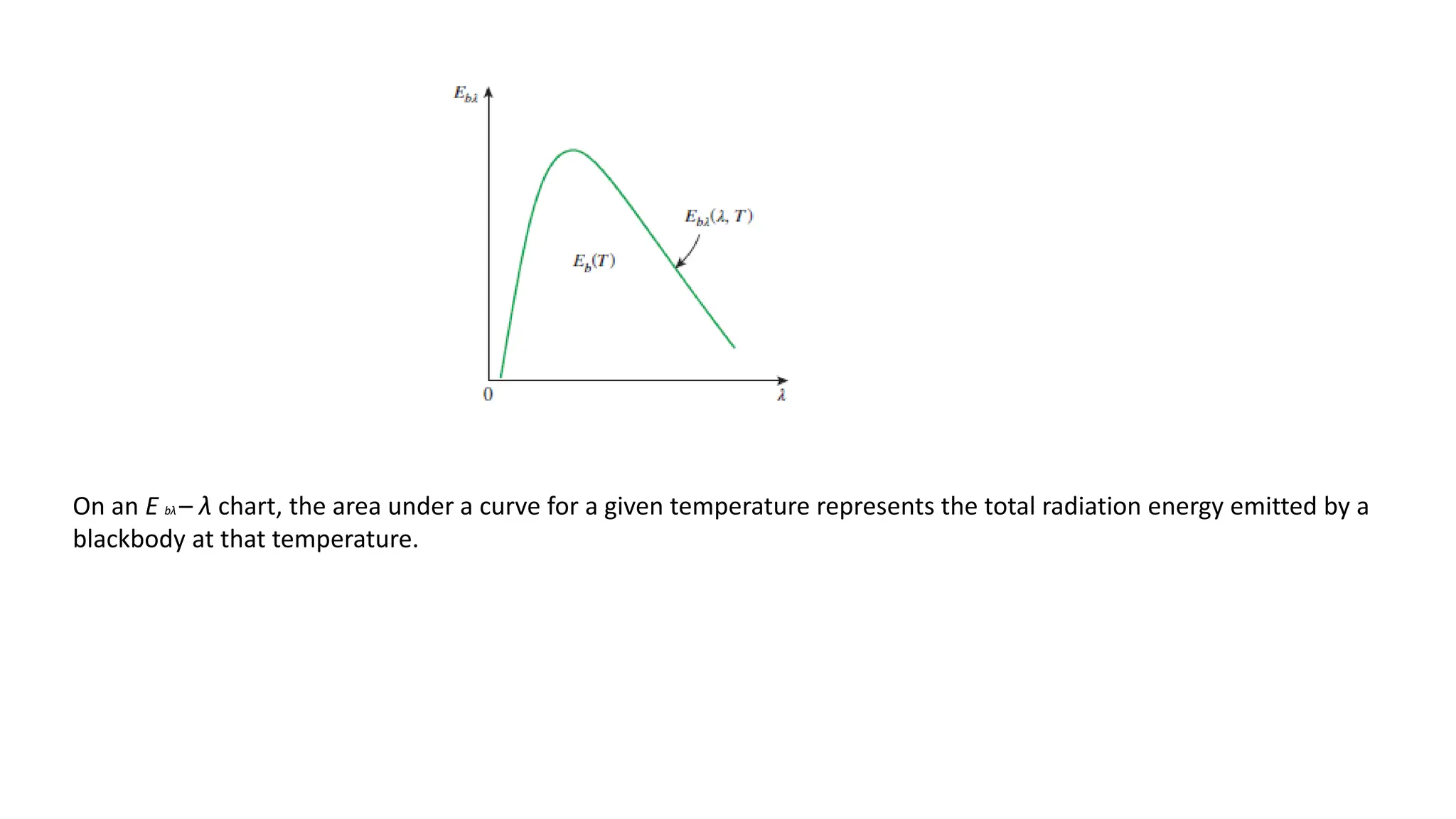
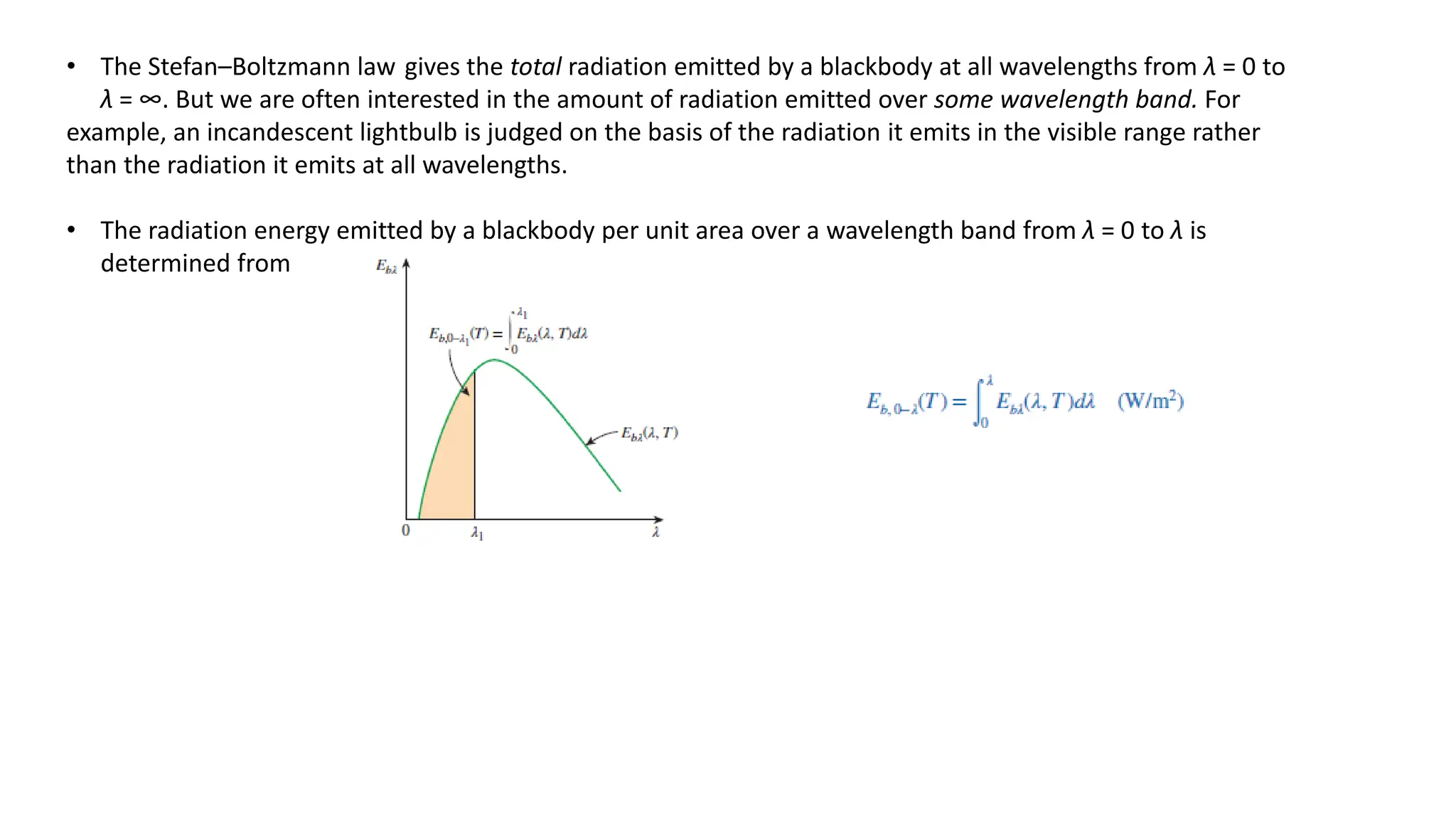
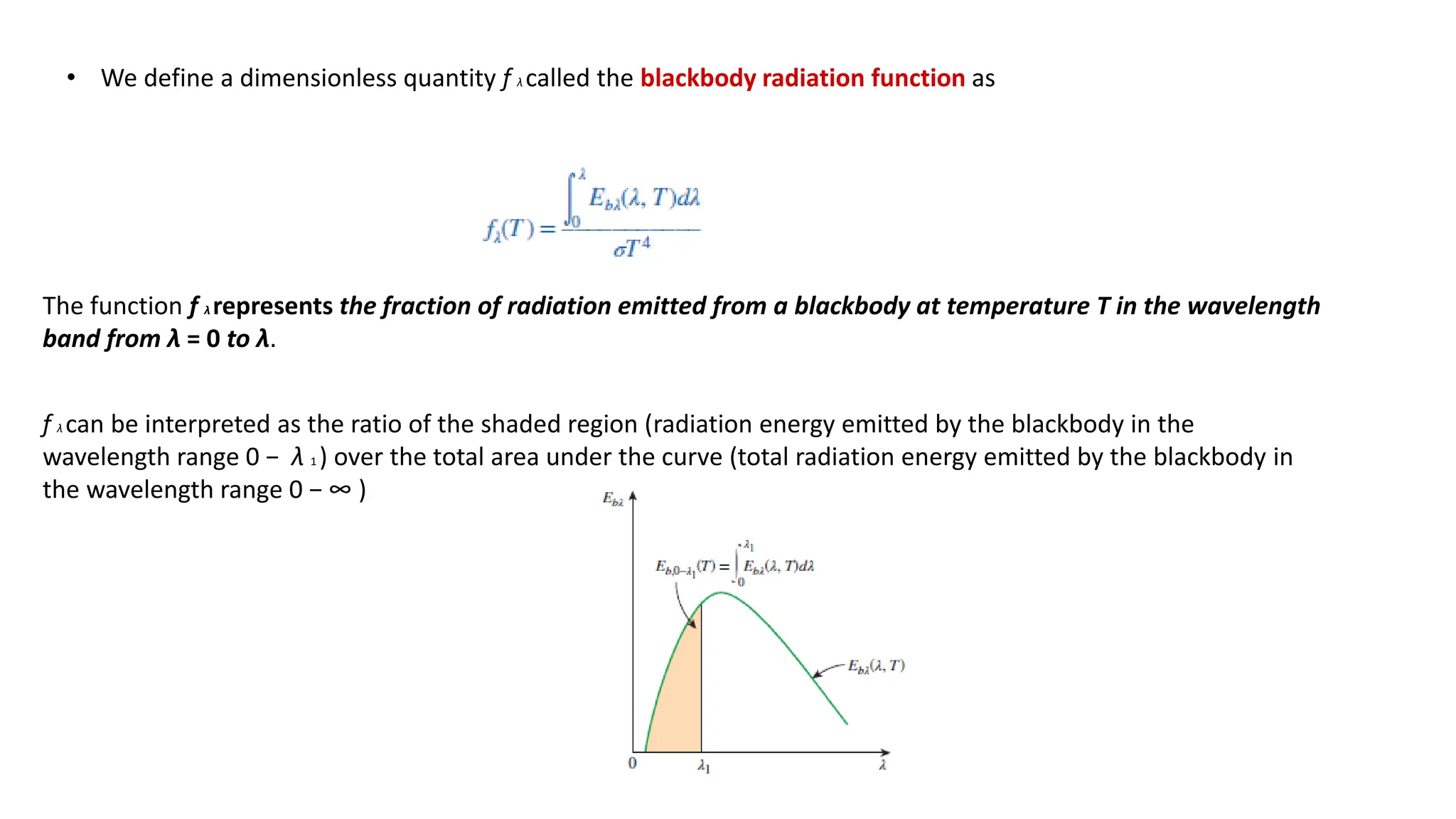
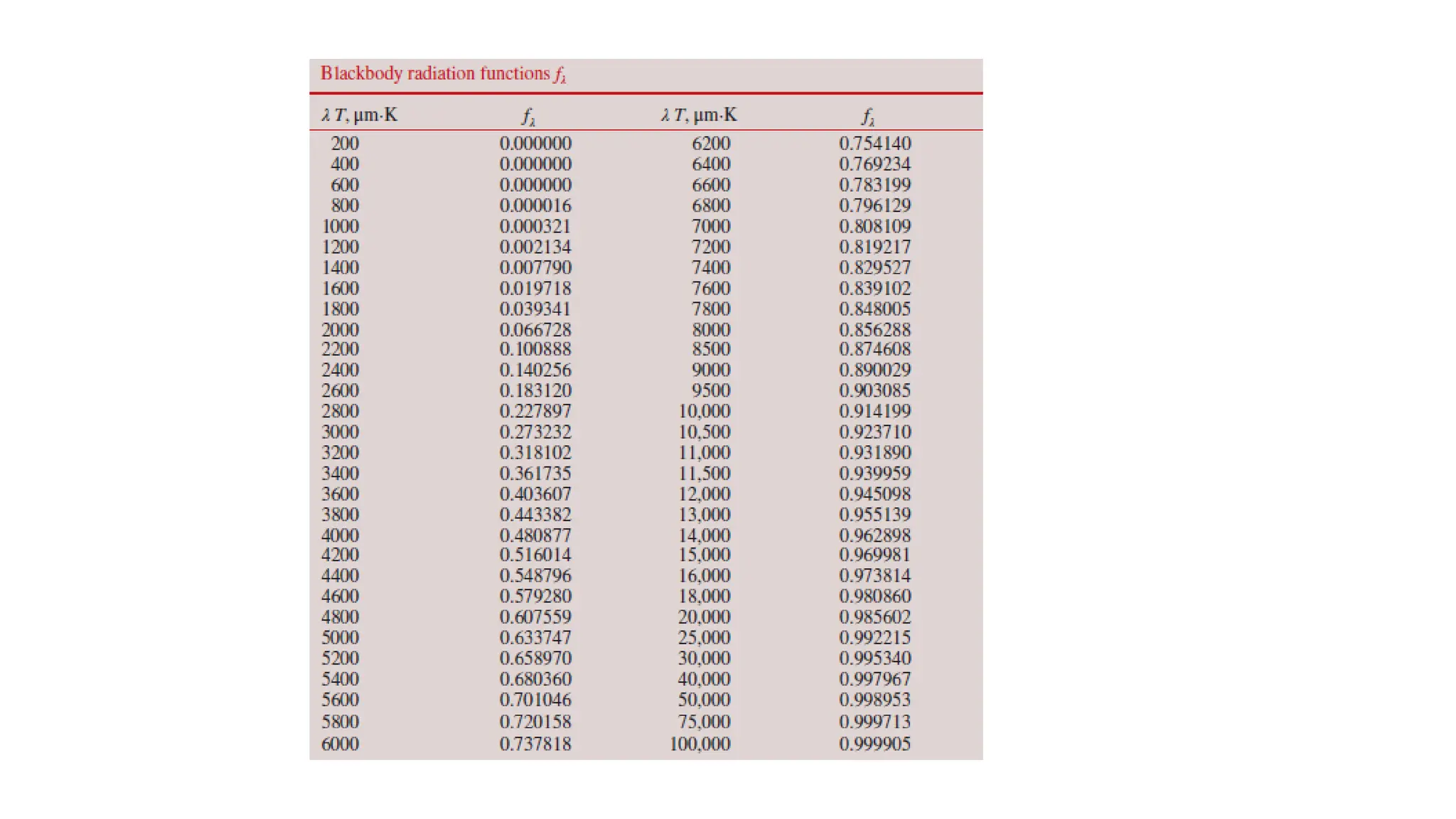
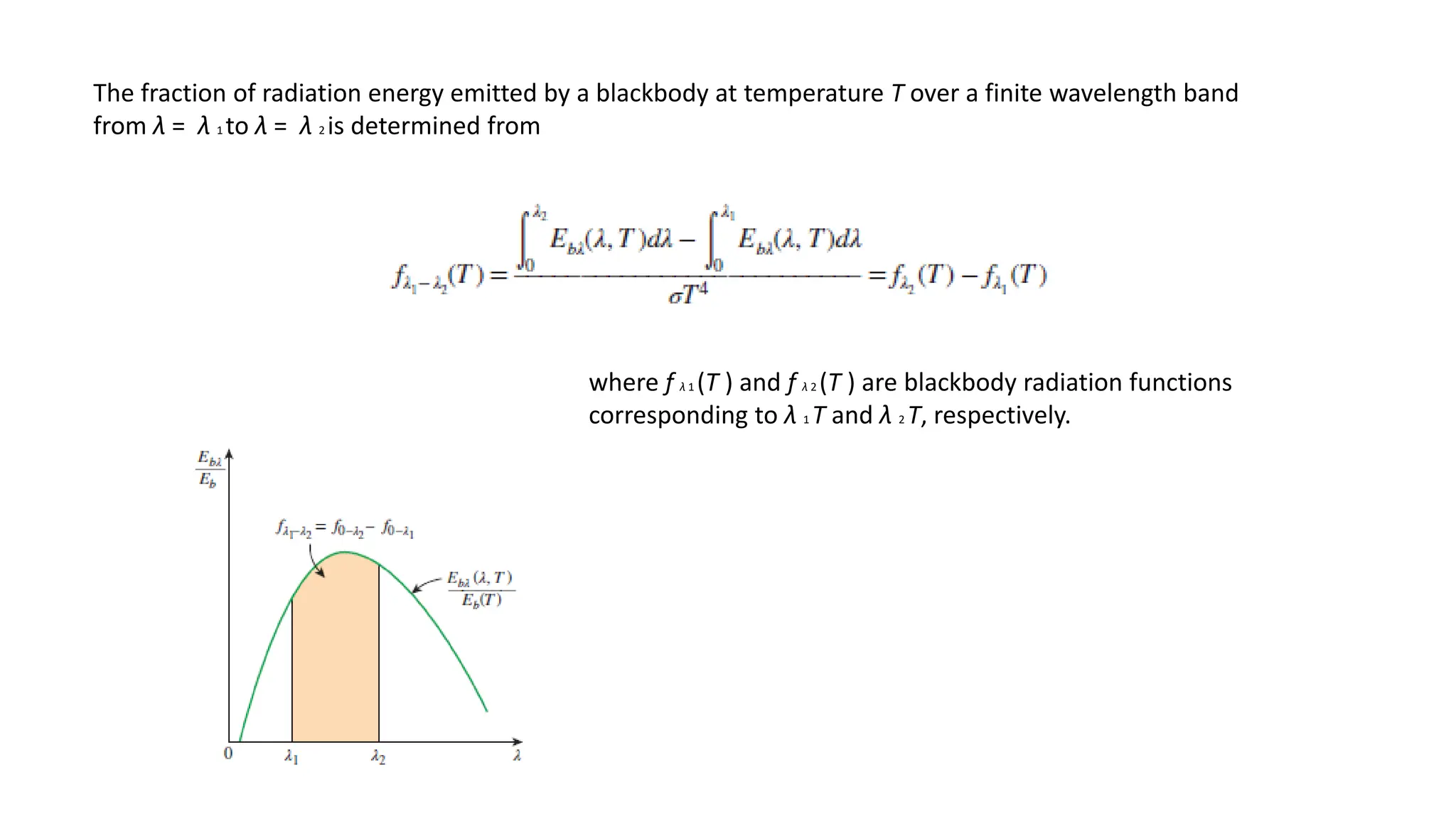
The document explains the mechanisms of heat transfer, particularly focusing on radiation, which occurs without a medium and is the fastest form of energy transfer. It discusses electromagnetic waves and thermal radiation, emphasizing the characteristics and significance of different wavelength ranges, particularly in relation to temperature and blackbody radiation. The Stefan-Boltzmann law and Planck’s law are highlighted as critical principles in understanding radiation emission and absorption across various materials.