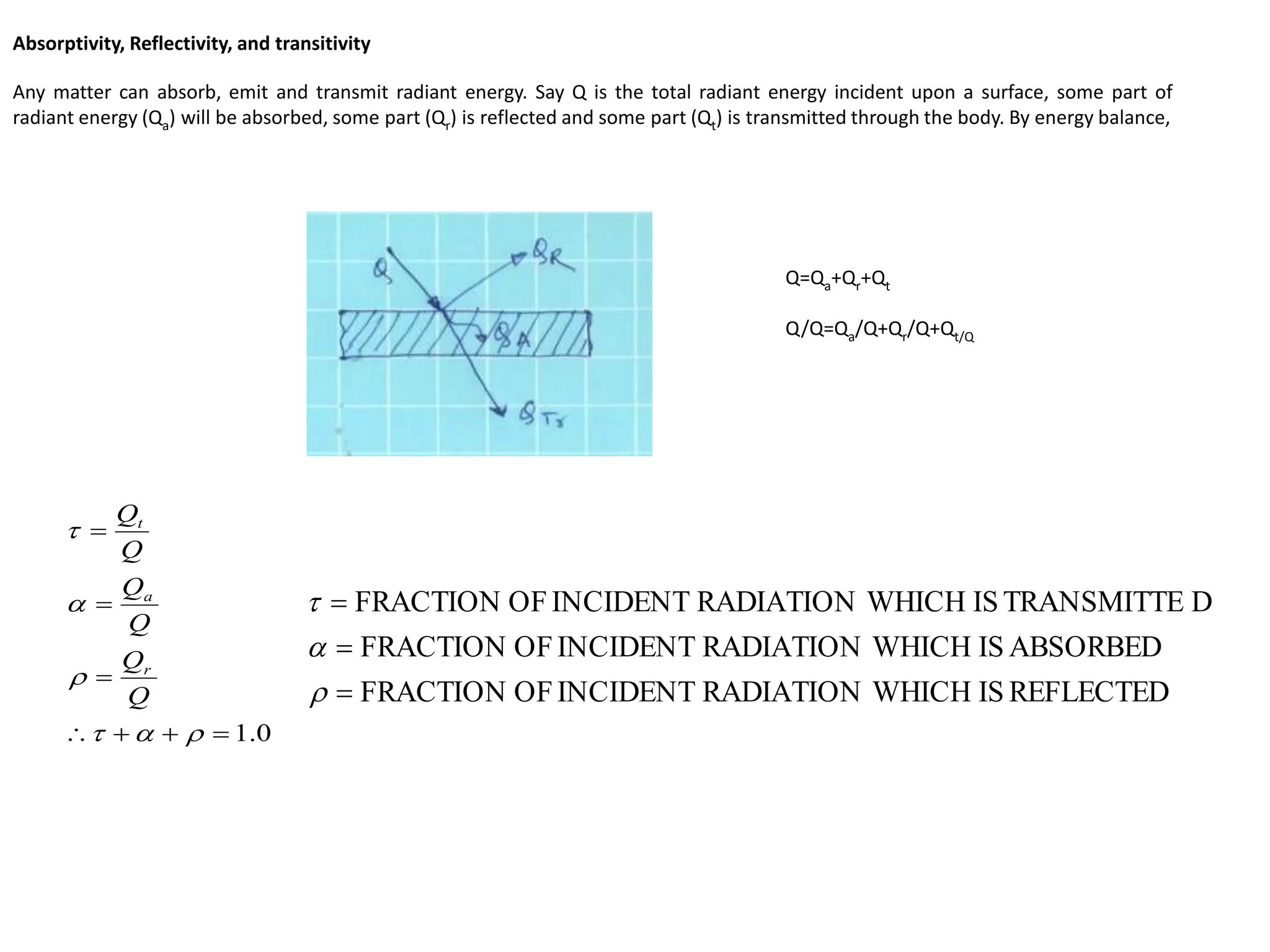
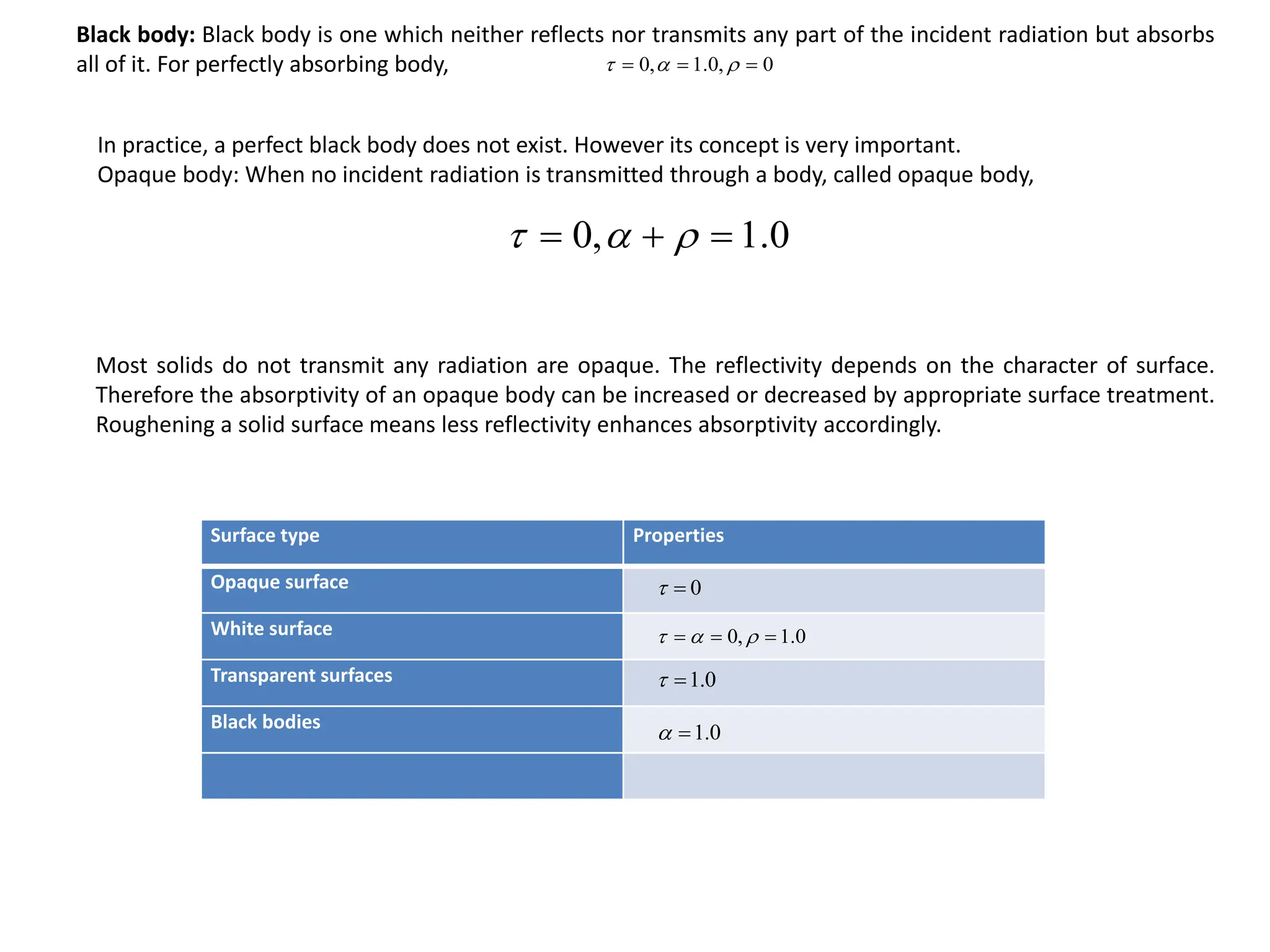
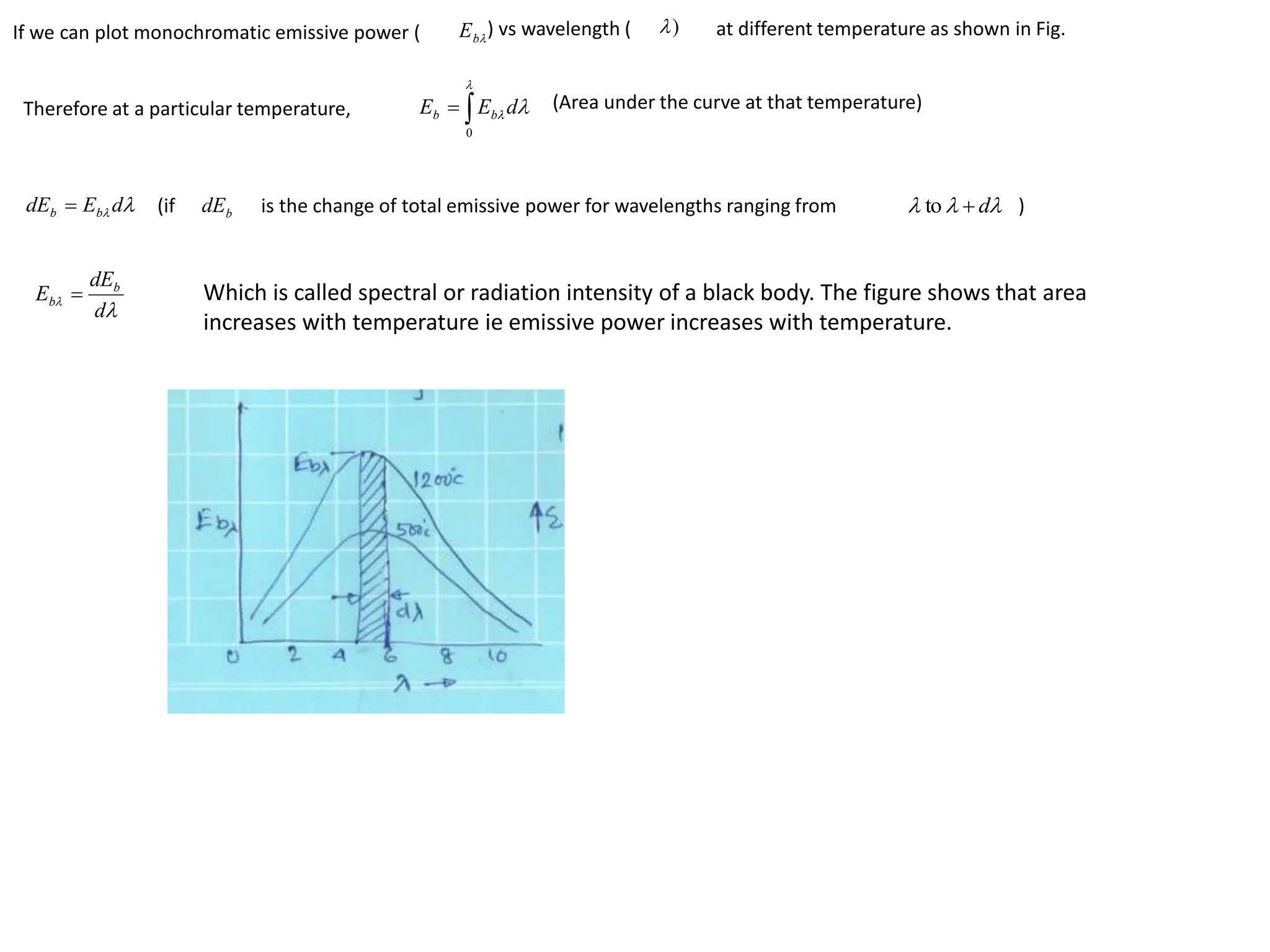
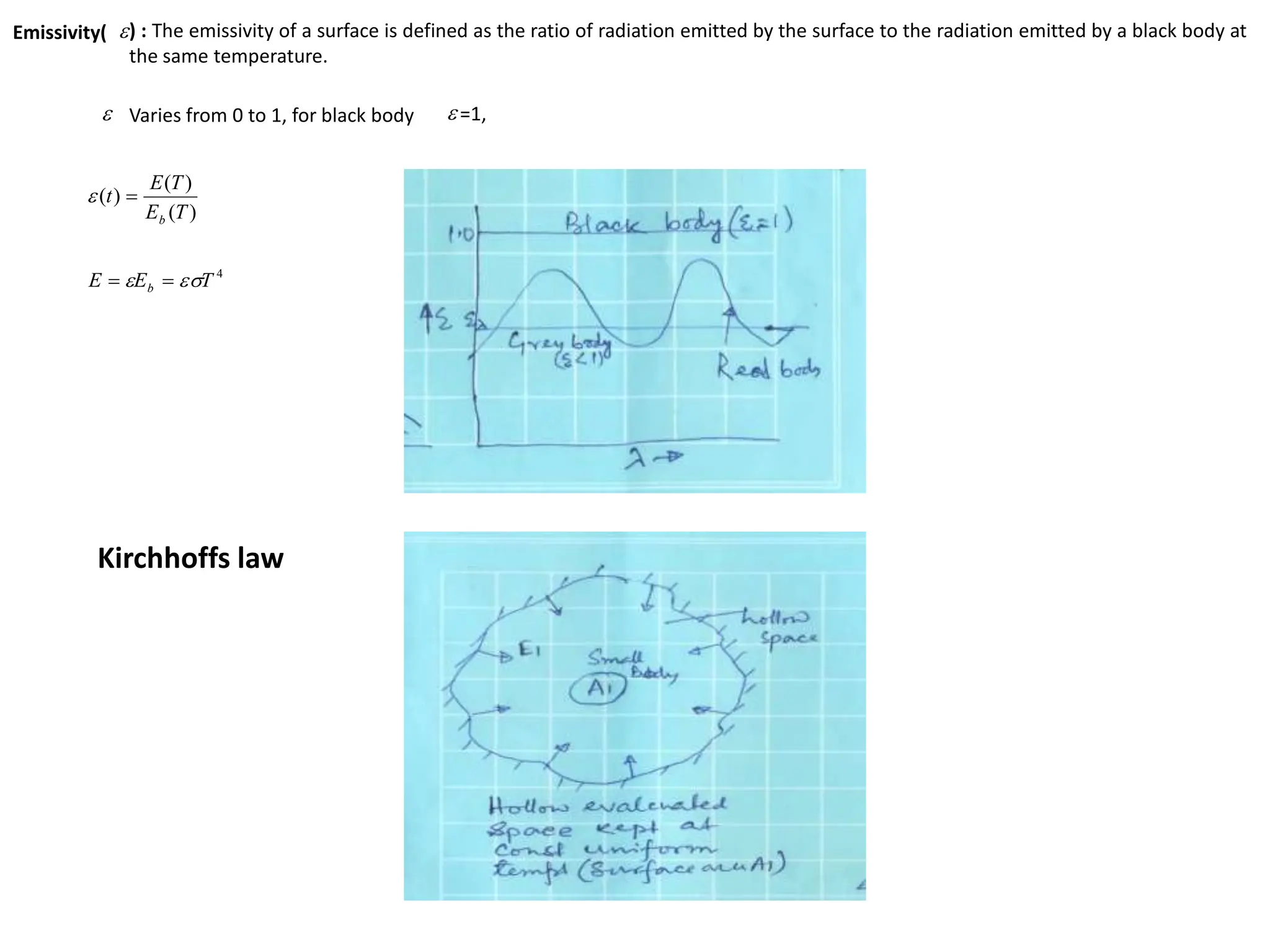
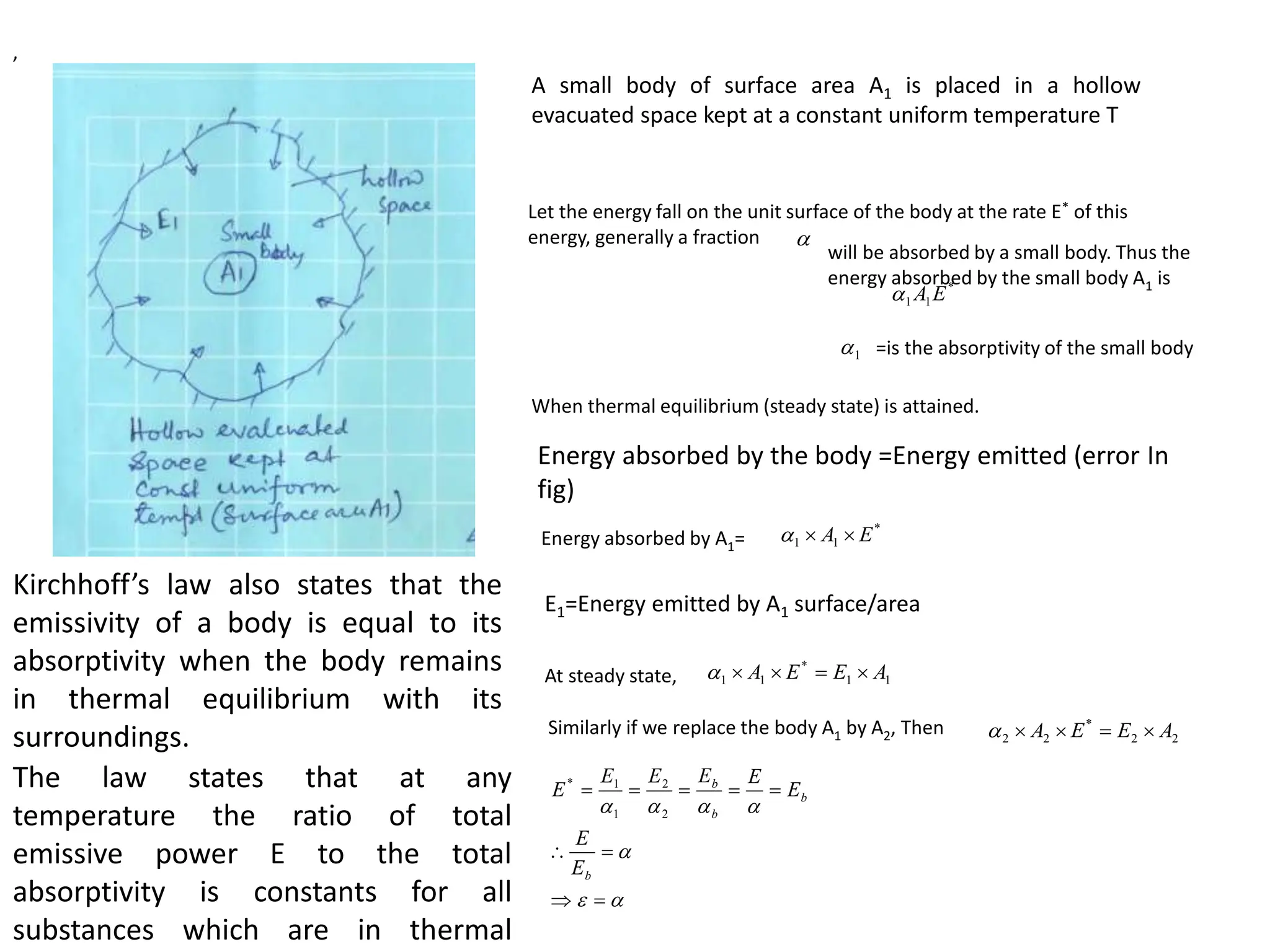
The document discusses fundamental concepts of radiation, including absorptivity, reflectivity, and transitivity of materials. It explains how a black body absorbs all incident radiation and introduces key laws like Stefan-Boltzmann law and Kirchhoff's law, emphasizing the relationship between emitted and absorbed radiation. Also mentioned are the concepts of emissive power, emissivity, and Wien's displacement law, which relate to the spectrum of thermal radiation emitted by bodies at different temperatures.