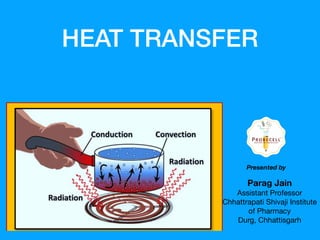
The document discusses the principles of heat transfer, detailing three basic mechanisms: conduction, convection, and radiation. It explains how heat flows from hot to cold areas, the mathematical formulation of heat transfer, and introduces the concept of heat exchangers, particularly the shell and tube types used in various applications. Additionally, it emphasizes the importance of temperature gradients in heat transfer and the efficiency of different heat exchanger designs.