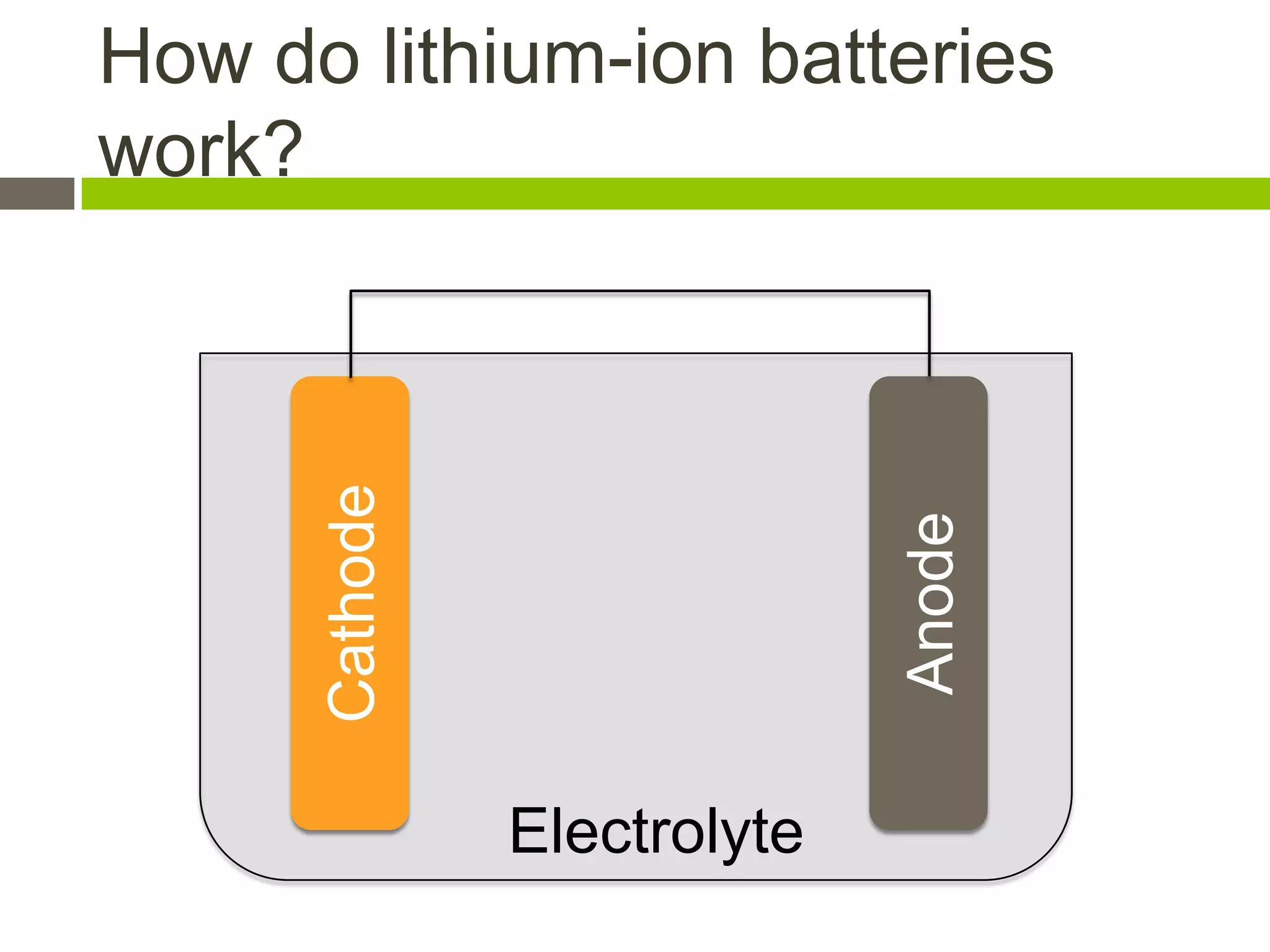
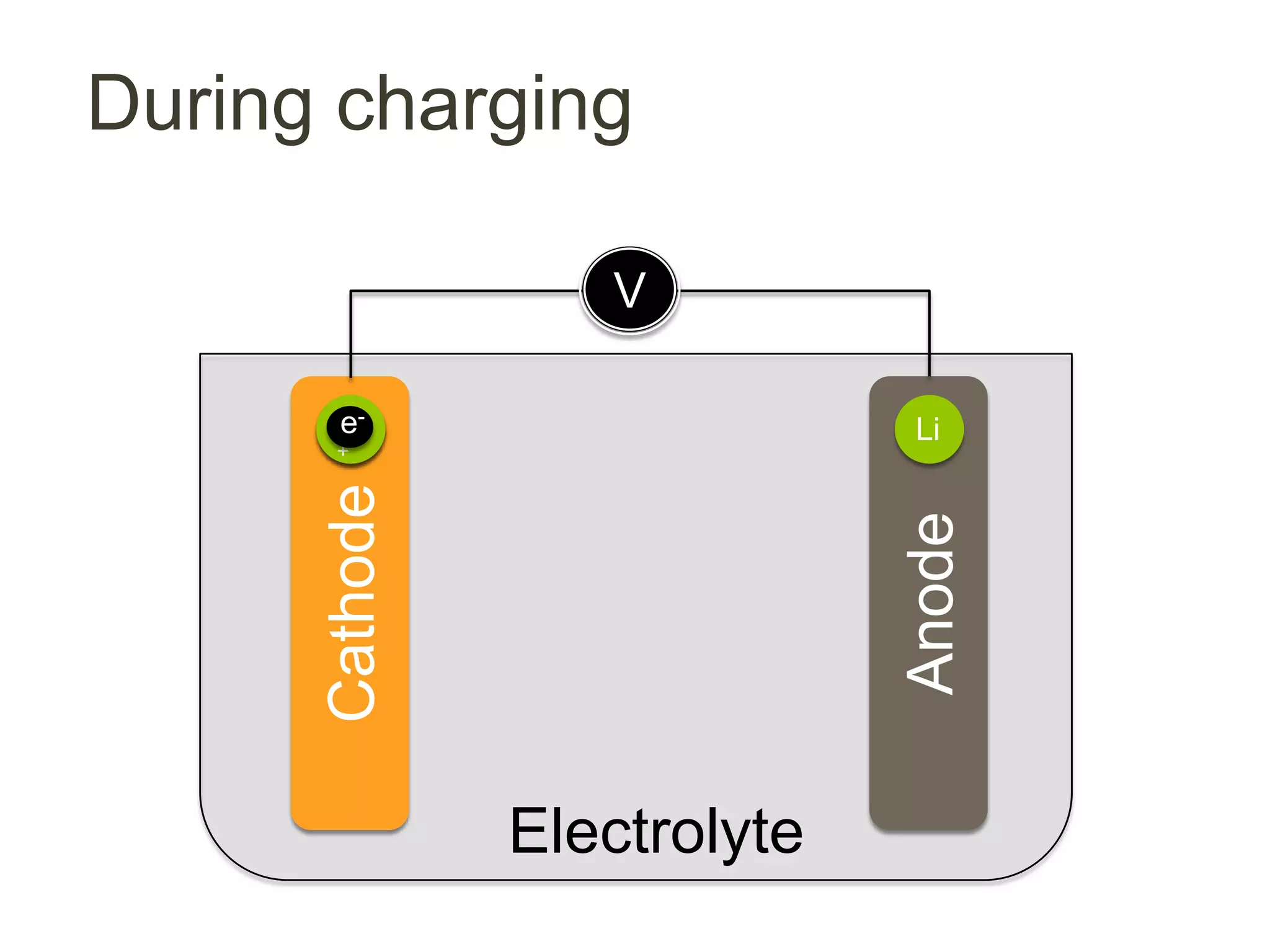
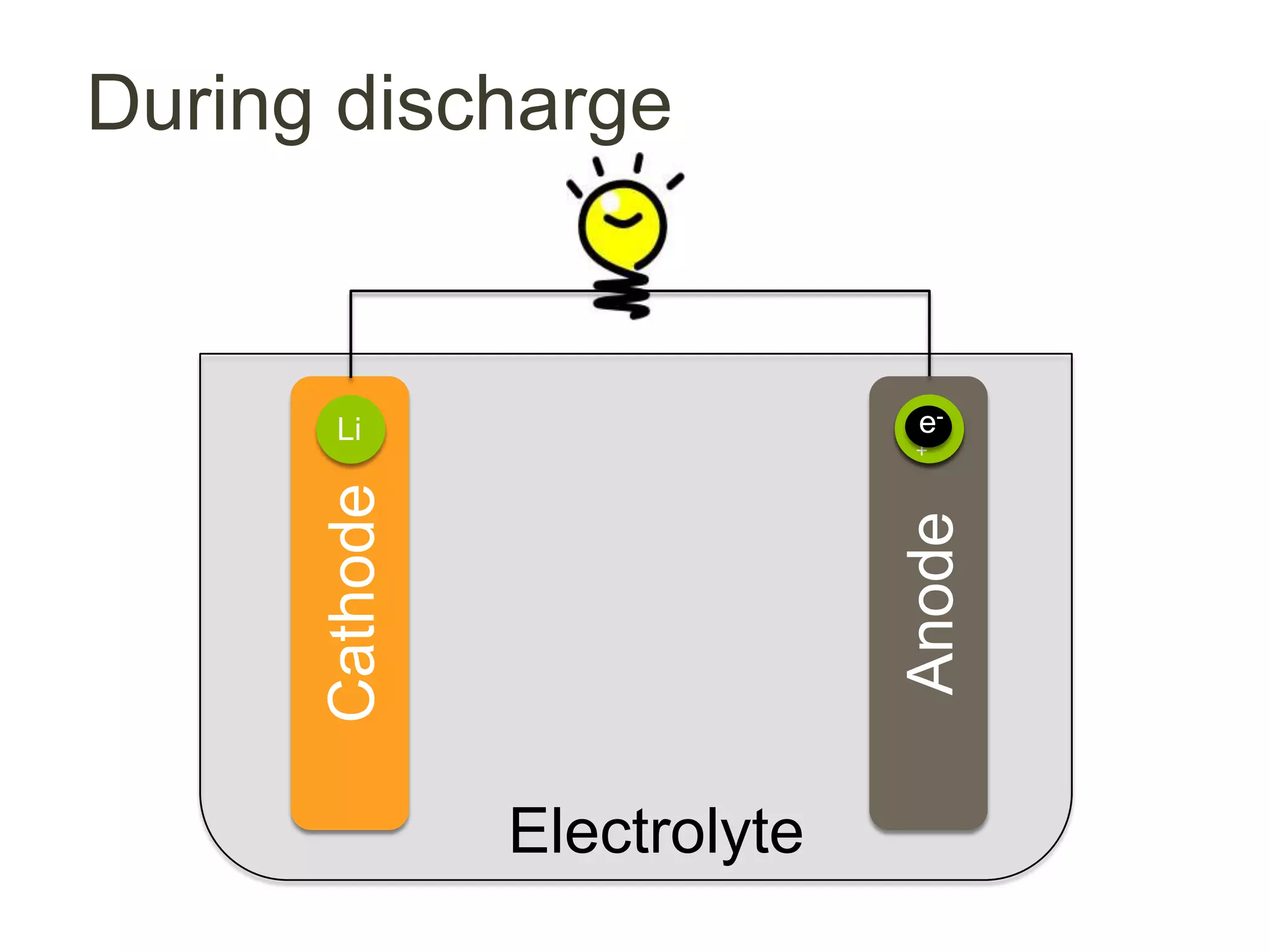
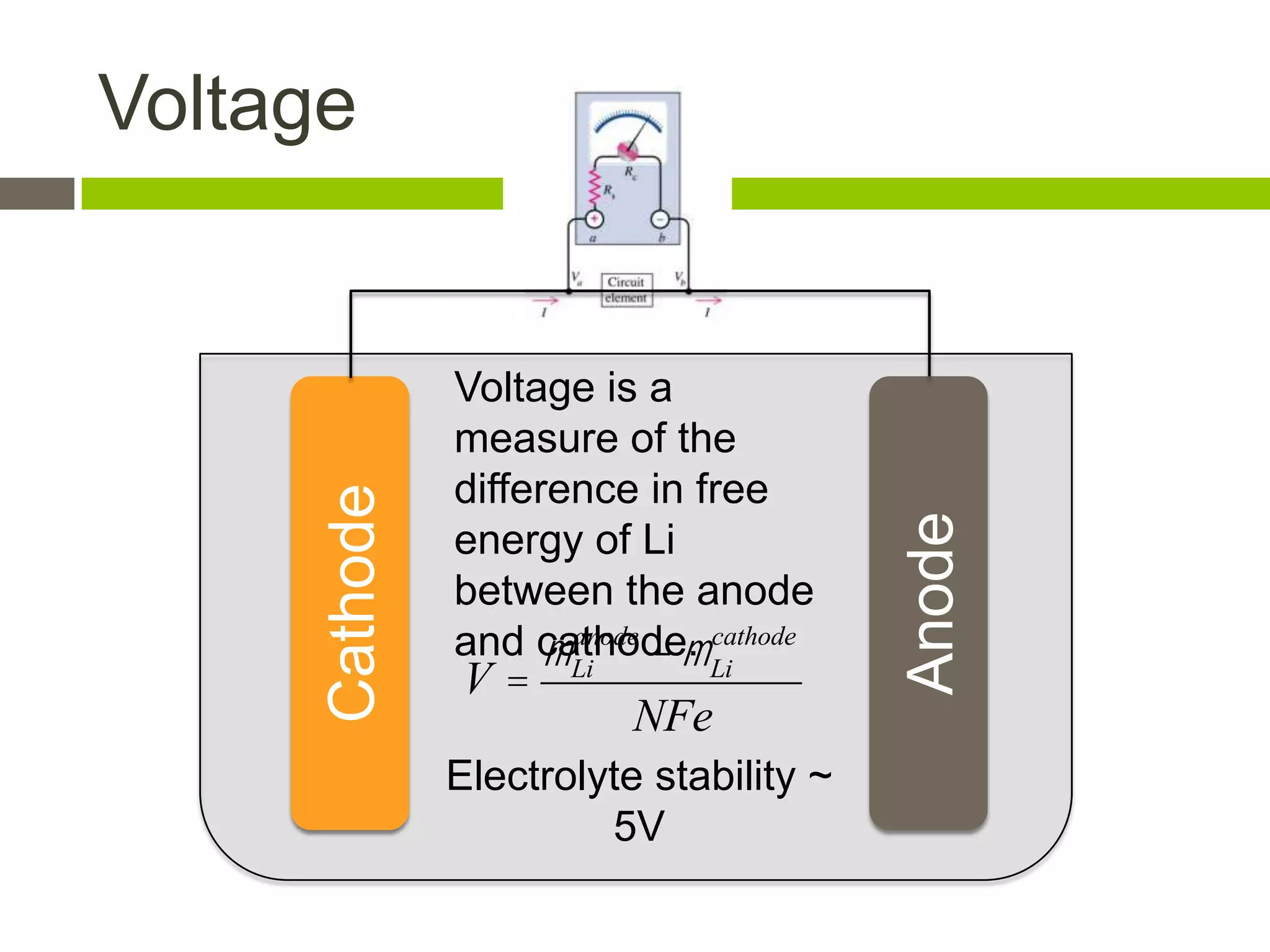
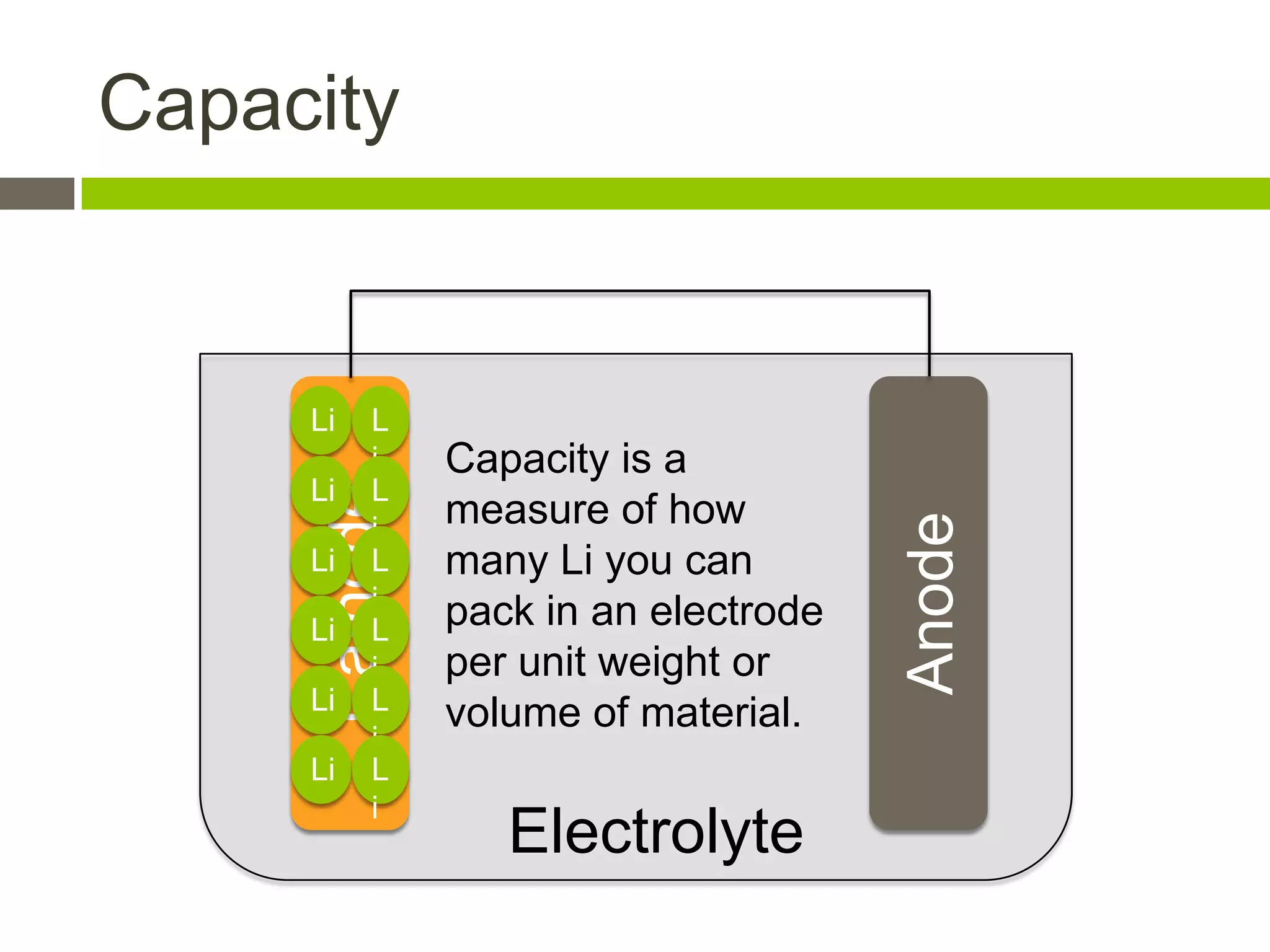
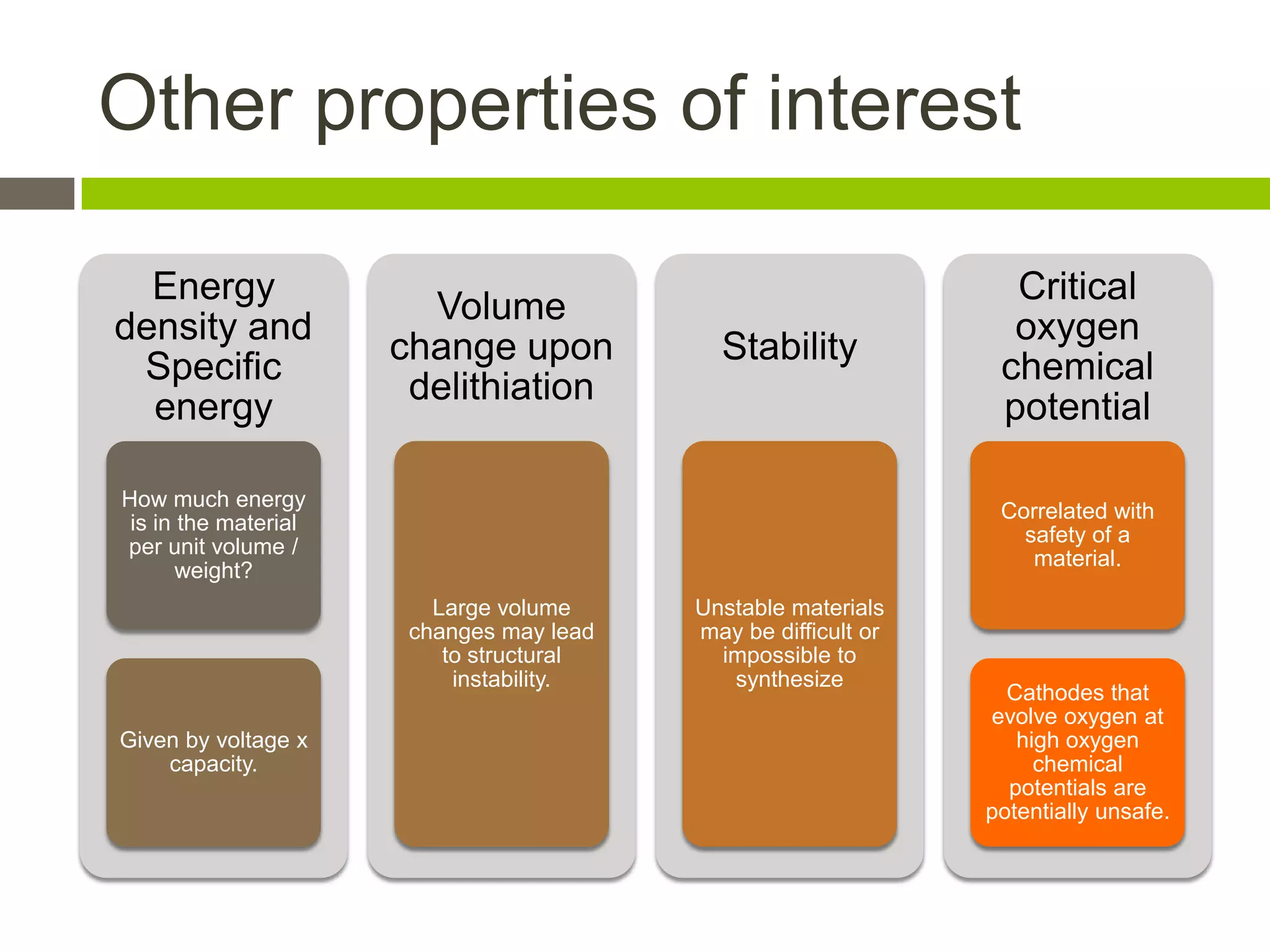
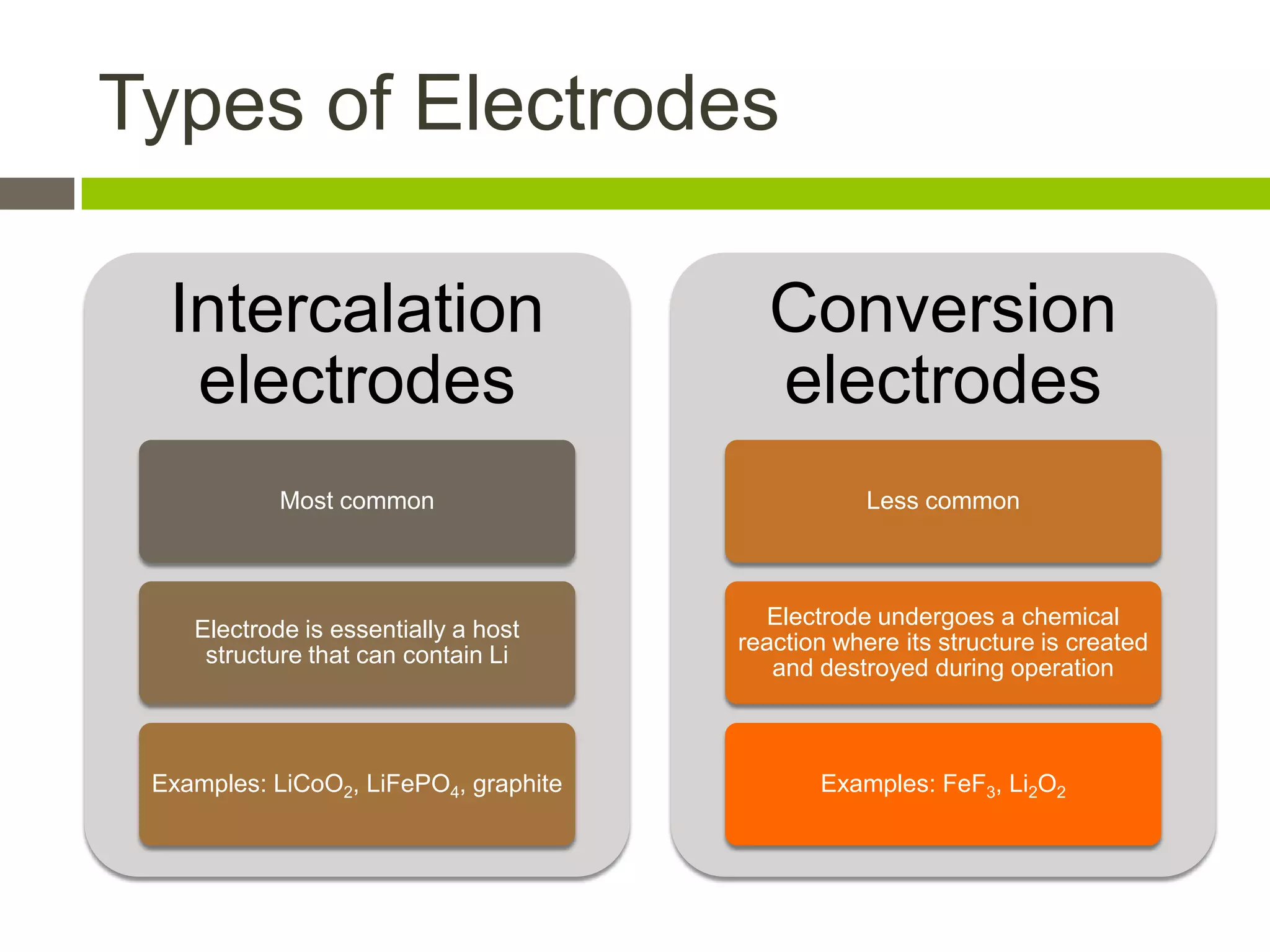
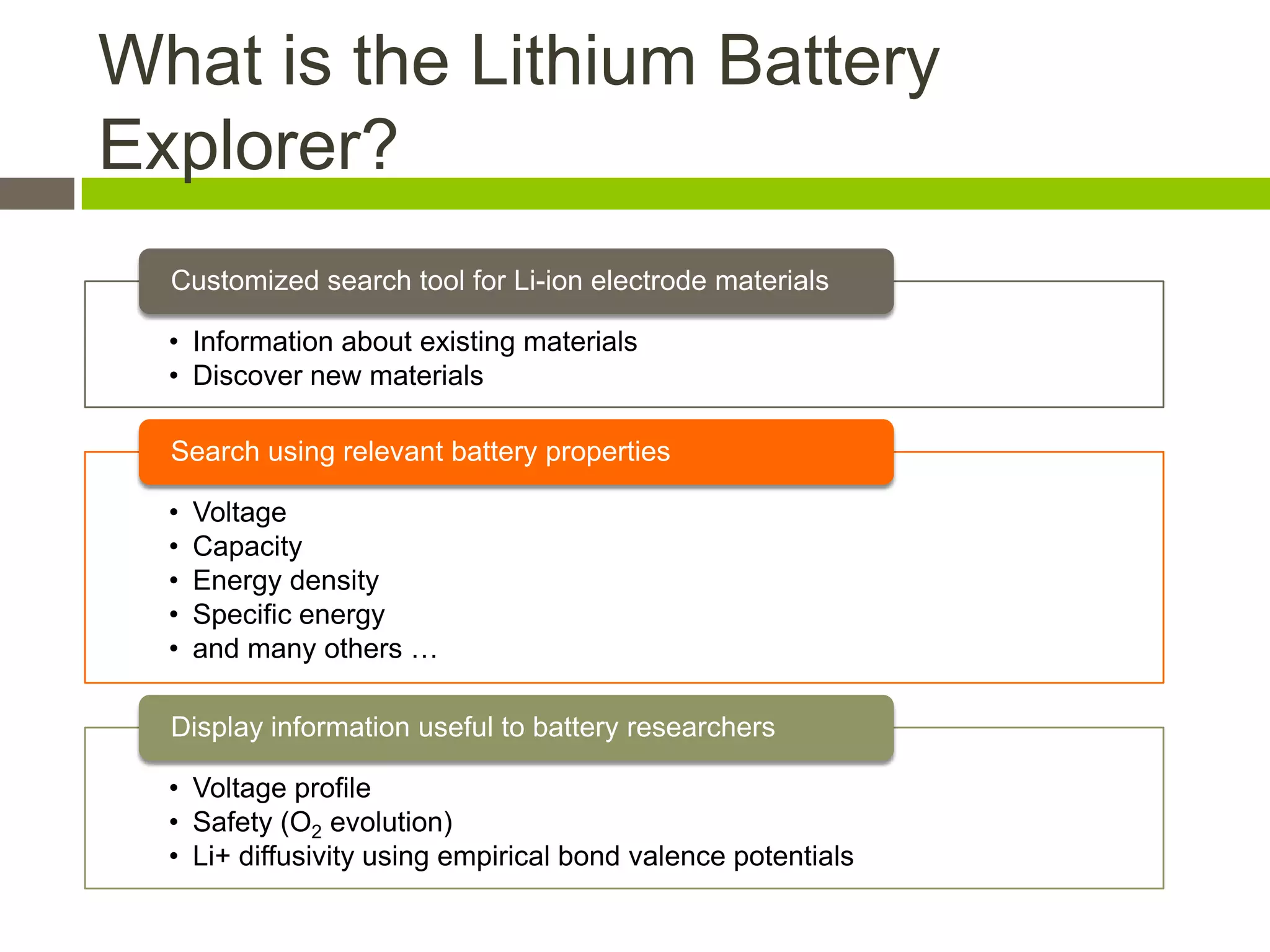
Lithium-ion batteries are rechargeable batteries with high energy density, widely used in portable electronics and increasingly in large-scale applications like hybrid electric vehicles. They work through the movement of lithium ions between the anode and cathode during charging and discharging, and key properties include voltage, capacity, energy density, and safety. The Lithium Battery Explorer is a custom search tool for finding information on lithium-ion electrode materials and their properties for battery research.