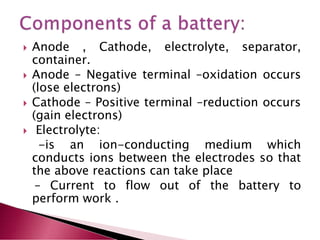
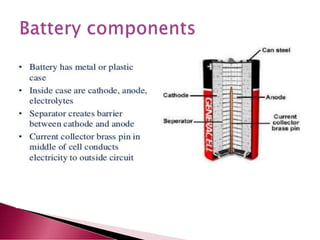
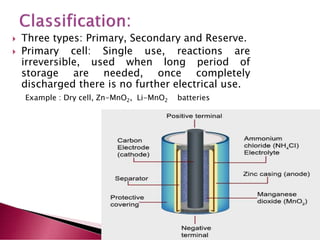
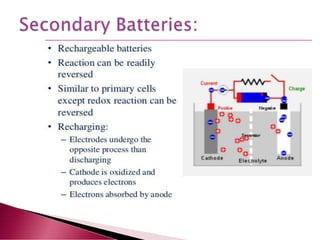
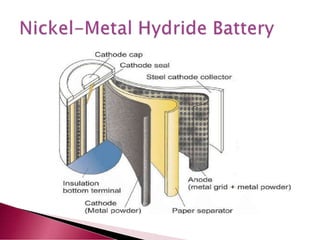
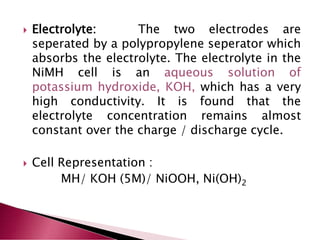
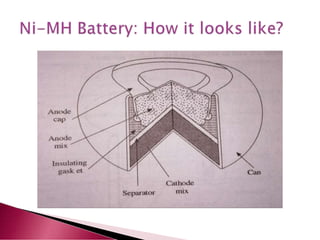
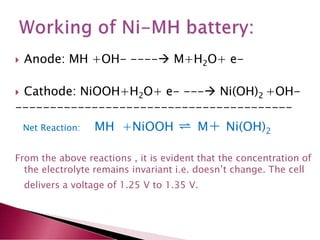
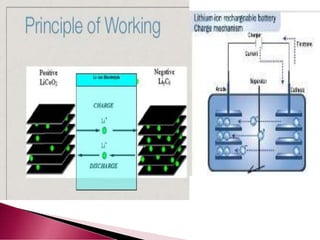
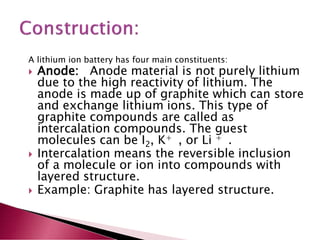
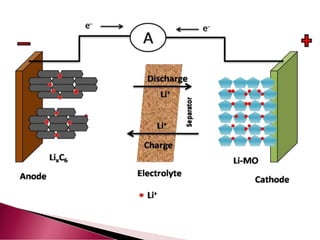
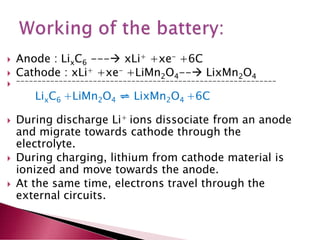
A lithium ion battery consists of a graphite anode, lithium metal oxide cathode, and electrolyte. Lithium ions move between the anode and cathode during charging and discharging, producing electricity. Key developments included the use of cobalt oxide and other metal oxides in the cathode by Goodenough, improving voltage and capacity. Lithium ion batteries now power many electronic devices due to their high energy density and ability to be recharged hundreds of times.