Embed presentation
Downloaded 369 times


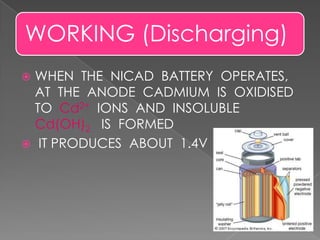



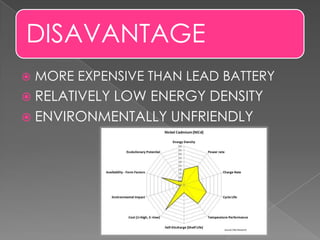





The document describes the composition and functioning of a nickel-cadmium (NiCad) battery. A NiCad battery consists of a cadmium anode, nickel oxide cathode, and potassium hydroxide electrolyte. During discharge, cadmium oxidizes to cadmium ions at the anode, producing around 1.4 volts. Recharging works by passing current in the opposite direction, reversing the electrode reactions and redepositing cadmium and nickel oxide. NiCad batteries provide fast charging, good load performance, and are smaller and lighter than lead batteries, though they are more expensive and have lower energy density.











