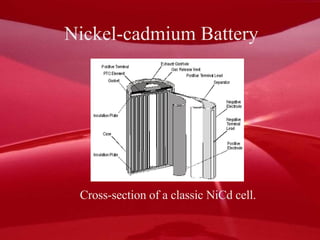
There are several main types of rechargeable batteries. Lead-acid batteries use lead and lead-oxide electrodes and sulfuric acid electrolyte; they are commonly used in cars. Nickel-cadmium batteries contain nickel-hydroxide and cadmium electrodes with potassium hydroxide electrolyte. Nickel-metal hydride batteries are similar but do not contain cadmium. Lithium-ion batteries have carbon anodes and metal oxide cathodes with an organic electrolyte, and are used in devices like laptops and phones. Rechargeable batteries can be restored to full charge through applying electrical energy to reverse the chemical reactions.