This document discusses materials used in batteries. It begins by introducing primary batteries such as zinc-carbon and alkaline batteries. It describes their characteristics and applications. Secondary batteries like lead-acid, nickel-cadmium, nickel-metal hydride, and lithium-ion batteries are then discussed, outlining their chemistries, characteristics, and uses. The document also provides a case study on the processing of lithium-ion batteries, describing steps such as mixing materials, coating electrodes, compression, drying, assembly, electrolyte filling, formation, grading, and packaging. Key materials used in batteries like various cathode and anode materials are also summarized.




![Primary Batteries
System Characteristics Applications
Zinc-carbon
(Leclanché), Zinc/MnO2
Common, low-cost primary battery; available in a
variety of sizes
Flashlight, portable radios, toys, novelties, instruments
Magnesium (Mg/MnO2) High-capacity primary battery; long shelf life Formerly used for military receiver-transmitters, and aircraft
emergency transmitters (EPIRBs)
Mercury (Zn/HgO) Highest capacity (by volume) of conventional types;
flat discharge; good shelf life
Hearing aids, medical devices (pacemakers), photography,
detectors, military equipment, but in limited use at present
due to environmental hazard of mercury
Mer-cad (Cd/HgO) Long shelf life; good low- and high-temperature
performance; low energy density
Special applications requiring operation under extreme
temperature conditions and long life; in limited use
Alkaline
(Zn/alkaline/MnO2)
Most popular general-purpose battery; good low-
temperature and high-rate performance; low cost
Most popular primary battery; used in a variety of
portable battery operated equipment
Lithium/ soluble
cathode
High energy density; long shelf life; good
performance over wide temperature range
Wide range of applications requiring high energy density,
long shelf life, e.g., from utility meters to military electronics
applications
Lithium/ solid cathode High energy density; good rate capability and low-
temperature performance; long shelf life;
competitive cost
Replacement for conventional button and cylindrical cell
applications, such as digital cameras
Lithium/ solid
electrolyte
Extremely long shelf life; low-power battery Medical electronics
Table 1: Characteristics and applications [1]](https://image.slidesharecdn.com/batterymaterialsreportgroup5final-150413170642-conversion-gate01/85/Battery-materials-6-320.jpg)
![Magnesium Batteries
Twice the service life or capacity of zinc battery
Disadvantages – voltage delay and parasitic corrosion
Potential > 2.8V, but 1.1V is achieved
Battery chemistry, Mg + 2 MnO2 + H2O Mn2O3 + Mg (OH) 2
Figure represents
Magnesium batteries
[2]](https://image.slidesharecdn.com/batterymaterialsreportgroup5final-150413170642-conversion-gate01/85/Battery-materials-7-320.jpg)
![Zinc Carbon batteries
Leclanché and zinc chloride systems
low cost, ready availability, and acceptable performance
Electrolyte – Ammonium chloride and zinc chloride
Carbions with Mg2O- Conductivity
Specific capacity- 75-35 A h/kg
Basic chemistry Zn + 2MnO2 ZnO.Mn2O3
Figure represents Zinc-Carbon
batteries
[3]](https://image.slidesharecdn.com/batterymaterialsreportgroup5final-150413170642-conversion-gate01/85/Battery-materials-8-320.jpg)

![Secondary Batteries [4]
System Characteristics Applications
LEAD-ACID:
Automotive Popular, low-cost secondary battery, low
specific-energy, high-rate, and low-
temperature performance; maintenance-
free designs
Automotive SLI, golf carts, lawn mowers, tractors, aircraft,
marine, micro-hybrid vehicles
Traction (motive power) Designed for deep 6-9 h discharge,
cycling service
Industrial trucks, materials handling, electric and hybrid
electric vehicles, special types for submarine power
Stationary Designed for standby float service, long
life, VRLA designs
Emergency power, utilities, telephone, UPS, load levelling,
energy storage, emergency lighting
Portable Sealed, maintenance-free, low cost, good
float capability, moderate cycle life
Portable tools, small appliances and devices, portable
electronic equipment
NICKEL-CADMIUM:
Industrial and FNC Good high-rate, low-temperature
capability, flat voltage, excellent cycle life
Aircraft batteries, industrial and emergency power
applications, communication equipment
Portable Sealed, maintenance-free, good high-rate
low-temperature performance, good
cycle life
Consumer electronics, portable tools, pagers, appliances,
photographic equipment, standby power, memory
backup
NICKEL-METAL
HYDRIDE
Sealed, maintenance-free, higher
capacity than nickel-cadmium batteries;
high energy density and power
Consumer electronics and other portable applications;
hybrid electric vehicles
LITHIUM-ION High specific energy and energy density,
long cycle life; high-power capability
Portable and consumer electronic equipment, electric
vehicles (EVs, HEVs, PHEVs), space applications, electrical
energy storage](https://image.slidesharecdn.com/batterymaterialsreportgroup5final-150413170642-conversion-gate01/85/Battery-materials-10-320.jpg)

![Construction of battery
Considering Aircraft battery design consists of steel case containing
identical, individual cells connected in series
And the end of the cells of the series are connected to receptacle located
on the outside of the case
[5] [6]](https://image.slidesharecdn.com/batterymaterialsreportgroup5final-150413170642-conversion-gate01/85/Battery-materials-13-320.jpg)
![Lithium Ion Batteries
Li ions exchange between the positive and negative
electrodes
The major advantages are they are sealed and no
maintenance is required, they have long life cycle, they
have long shelf life, and low self-discharge rate. High power
discharge rate capability
The major disadvantages are that, they degrade at high
temperatures, capacity loss and potential for thermal
runway when charged, possible venting and possible
thermal runway when crushed, and may become unsafe
when rapid charge at low temperature (< 0 0C).
higher specific energy (up to 240 Wh/kg)
energy density (up to 640 Wh/L)
self-discharge rate is around 2-8% per month
The working temperature range is at 0 to 45 0C
Single cell Operating Voltage between 2.5 and 4.3 V
[7]](https://image.slidesharecdn.com/batterymaterialsreportgroup5final-150413170642-conversion-gate01/85/Battery-materials-14-320.jpg)

![Battery materials [7]
Material
Specific
capacity
mAh/g
Comments
LiCoO2 155 Still the most common. Co is expensive.
LiNi1-x-yMnxCoyO2 (NMC) 140-180
Safer and less expensive than LiCoO2. Capacity depends on
upper voltage cut-off.
LiNi0.8Co0.15Al0.05O2 200 About as safe as LiCoO2, high capacity.
LiMn2O4 100-120
Inexpensive, safer than LiCoO2, poor high temperature stability
(but improving with R&D).
LiFePO4 160
Synthesis in inert gas leads to process cost. Very safe. Low
volumetric energy.
Li[Li1/9Ni1/3Mn5/9]O2 275 High specific capacity, R&D scale, low rate capability.
LiNi0.5Mn1.5O4 130 Requires an electrolyte that is stable at a high voltage.](https://image.slidesharecdn.com/batterymaterialsreportgroup5final-150413170642-conversion-gate01/85/Battery-materials-17-320.jpg)
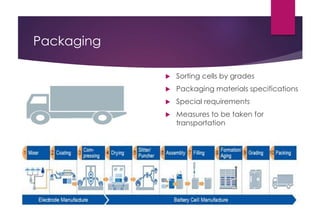
![Battery Manufacturing Process[11]](https://image.slidesharecdn.com/batterymaterialsreportgroup5final-150413170642-conversion-gate01/85/Battery-materials-19-320.jpg)


![REFERENCES
[1] Thomas Reddy. "Chapter 8 - An Introduction to Primary Batteries". Linden's Handbook of Batteries, Fourth
Edition.McGraw-Hill, © 2011. Books24x7. Web. Apr. 7, 2015. http://common.books24x7.com/toc.aspx?bookid=35916
[2] Thomas Reddy. "Chapter 10 - Magnesium and Aluminium Batteries". Linden's Handbook of Batteries, Fourth Edition.
McGraw-Hill. © 2011. Books24x7. http://common.books24x7.com/toc.aspx?bookid=35916 (accessed April 8, 2015)
[3] Thomas Reddy. "Chapter 9 - Zinc-Carbon Batteries—Leclanché and Zinc Chloride Cell Systems". Linden's Handbook of
Batteries, Fourth Edition. McGraw-Hill, © 2011.Books24x7.Web. Apr.7, 2015.
http://common.books24x7.com/toc.aspx?bookid=35916
[4] Thomas Reddy. "Chapter 15 - An Introduction to Secondary Batteries". Linden's Handbook of Batteries, Fourth
Edition. McGraw-Hill, © 2011. Books24x7. Web. Apr. 9, 2015.http://common.books24x7.com/toc.aspx?bookid=35916
[5] D. Vutetakis, “Batteries,” in Digital Avionics Handbook, Third Edition, CRC Press, 2014, pp. 419–442.
[6] Thomas Reddy. "Chapter 19 - Industrial and Aerospace Nickel-Cadmium Batteries". Linden's Handbook of Batteries,
Fourth Edition. McGraw-Hill, © 2011. Books24x7. Web. Apr. 9, 2015. http://common.books24x7.com/toc.aspx?bookid=35916
[7] Thomas Reddy. "Chapter 26 - Lithium-Ion Batteries”. Linden’s Handbook of Batteries, Fourth Edition. McGraw-Hill, ©
2011. Books24x7. Web. Apr. 9, 2015 http://common.books24x7.com/toc.aspx?bookid=35916
[8] A. Manthiram, “Smart Battery Materials,” in Smart Materials, CRC Press, 2008.
[9] D. Vutetakis, “Batteries,” in Digital Avionics Handbook, Third Edition, CRC Press, 2014, pp. 419–442.
[10] Z. Bakenov and I. Taniguchi, “Cathode Materials for Lithium-Ion Batteries,” in Lithium-Ion Batteries, CRC Press, 2011, pp.
51–96.
[11] http://www.industry.siemens.com/topics/global/en/battery-manufacturing/process/pages/default.aspx](https://image.slidesharecdn.com/batterymaterialsreportgroup5final-150413170642-conversion-gate01/85/Battery-materials-31-320.jpg)
