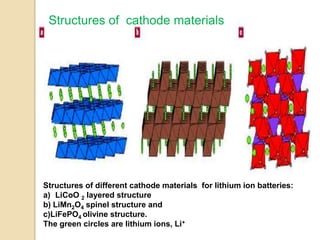
The document discusses the design of new cathode materials for secondary lithium ion batteries. It provides background on the development of batteries over time and describes the basic components and operation of lithium ion batteries. Current commercially used cathode materials like lithium cobalt oxide, lithium nickel oxide, lithium manganese oxide, and lithium iron phosphate are described. Research aims to develop new cathode materials with improved properties like higher energy density, longer lifespan, lower cost, and environmental friendliness. Promising candidates include olivine-based phosphates and transition metal oxides.