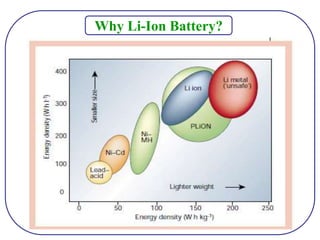
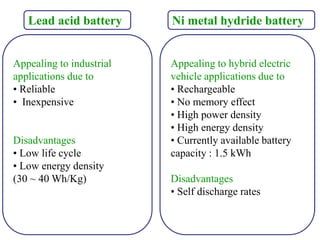
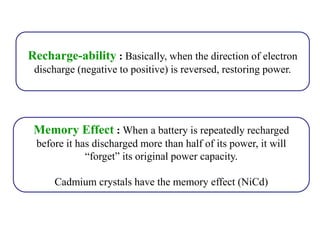
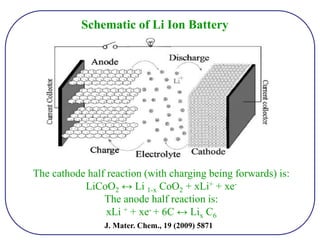
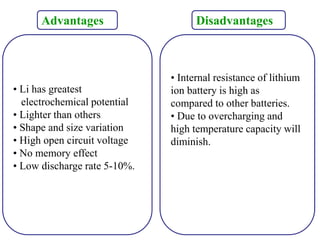
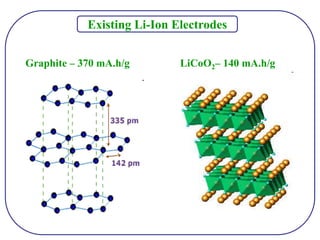
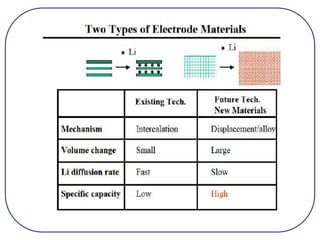
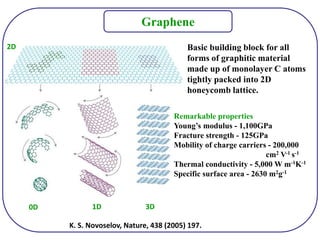
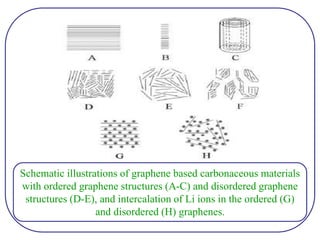
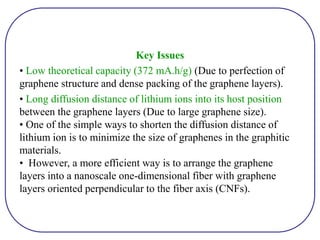
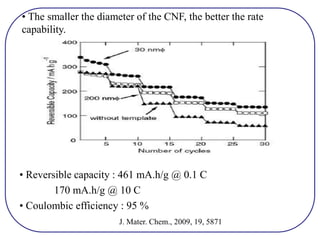
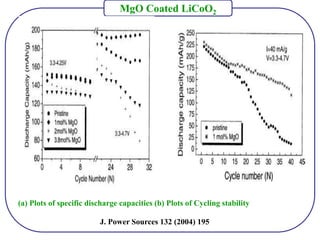
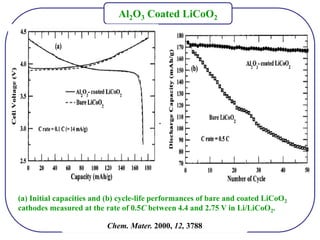
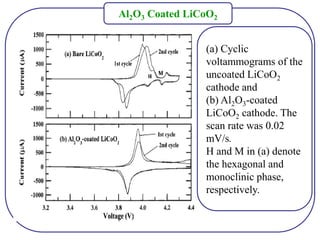
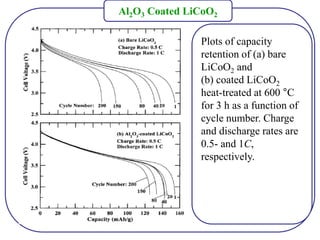
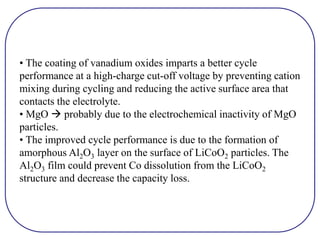
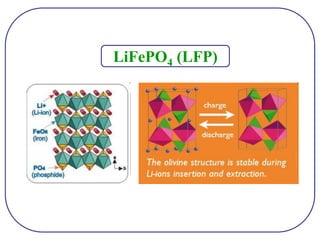
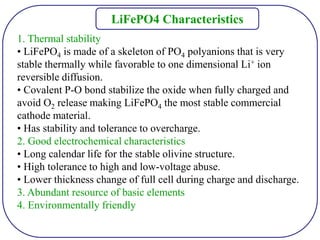
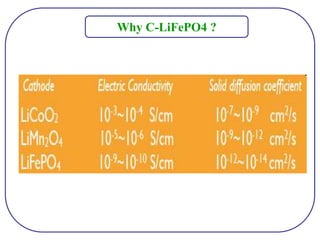
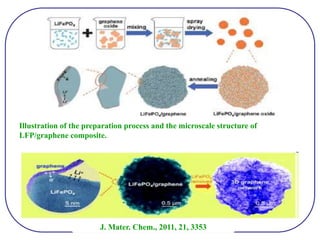
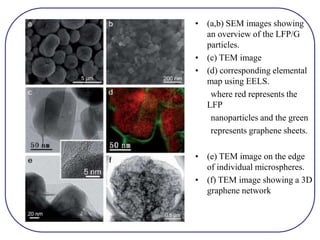
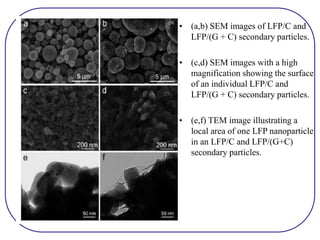
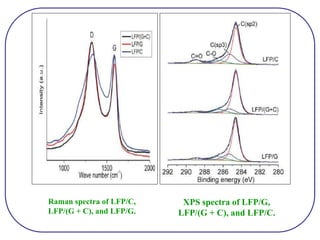
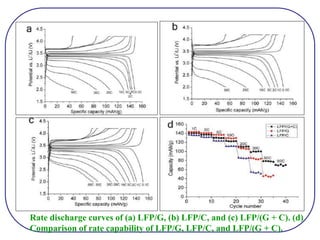
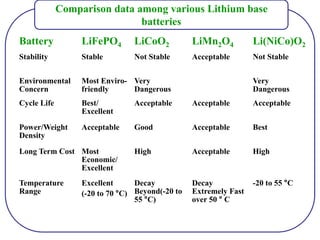
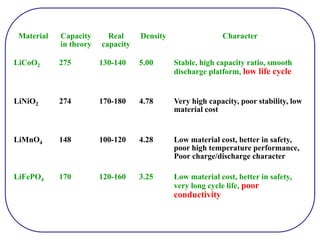
The document discusses lithium ion batteries and their components. It provides details on various cathode and anode materials used in lithium ion batteries, including their composition, advantages, and disadvantages. For cathodes, it describes lithium cobalt oxide (LiCoO2), lithium iron phosphate (LiFePO4), and coatings used to improve stability. For anodes, it discusses graphite and alternatives like silicon and tin, and ways to improve cycle life through nanostructuring and graphene composites. It also provides an overview of lithium ion battery operation and requirements for electrode materials.