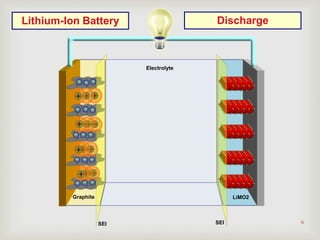
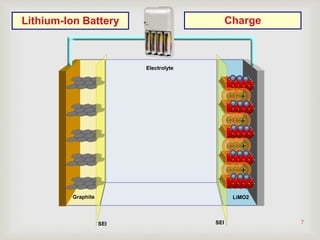
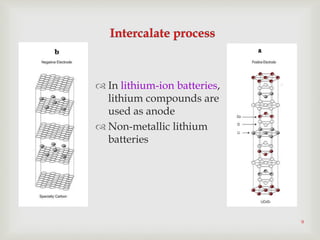
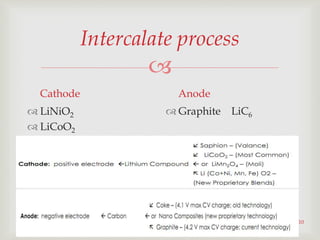
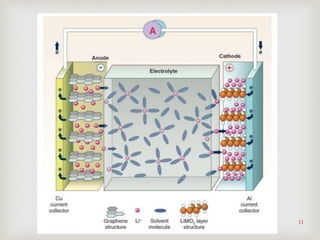
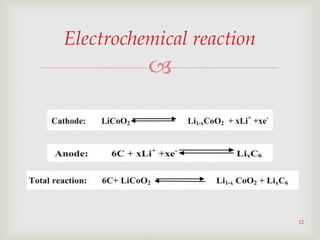
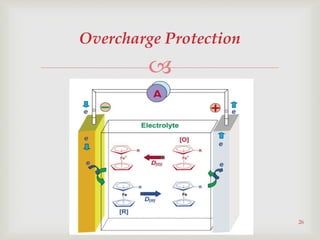
Lithium-ion batteries were first proposed in the 1970s but were not successfully created until the mid-1980s. The first commercial lithium-ion battery was launched by Sony in 1991. Lithium-ion batteries use lithium compounds in the anode and a lithium cobalt oxide or lithium iron phosphate cathode. During discharge, lithium ions move from the anode to the cathode and back during charging through an electrolyte. Lithium-ion batteries have a high energy density and output voltage, long cycle life, and are more environmentally friendly than alternatives. However, they are also more expensive and require temperature monitoring and sealing to prevent issues.



















![
40
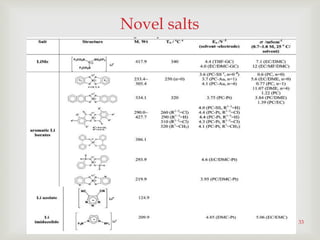
Li2B12F12-xHx-based electrolytes possess several
advantages over conventional LiPF6-based
electrolyte.
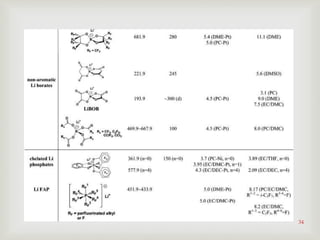
Graphite/Li1.1[Ni1/3Mn1/3Co1/3]0.9O2 cells using
Li2B12F9H3-based electrolyte, using lithium
difluoro(oxalato)borate as the electrolyte additive](https://image.slidesharecdn.com/lithiumionbatteries-170425080955/85/Lithium-ion-batteries-40-320.jpg)

