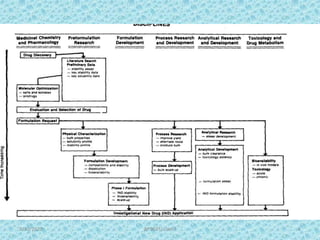
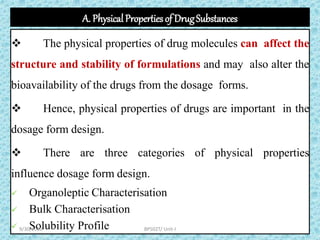
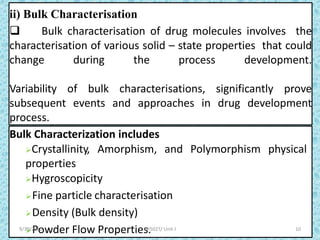
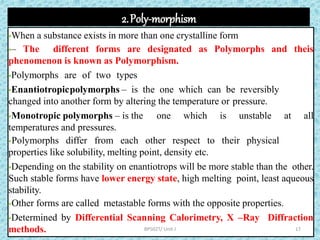
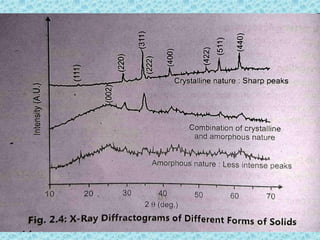
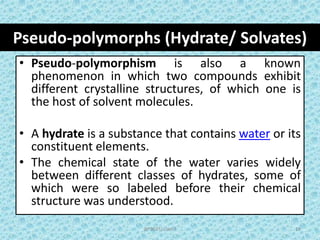
The document provides an introduction to preformulation, which involves studying the physical and chemical properties of a drug substance prior to formulation development. The goals of preformulation are to generate information to develop stable, bioavailable dosage forms and establish parameters that may affect drug performance. Key physical properties studied include organoleptic characteristics, bulk properties like solubility and polymorphism, and chemical properties like hydrolysis and oxidation. Understanding these characteristics is essential for designing optimal drug delivery systems. Preformulation is the first step in rational development of a dosage form.