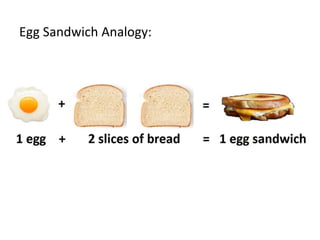
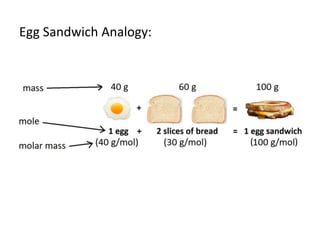
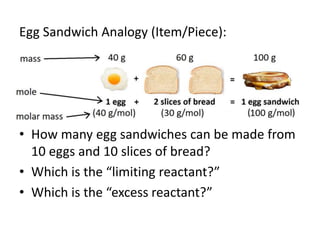
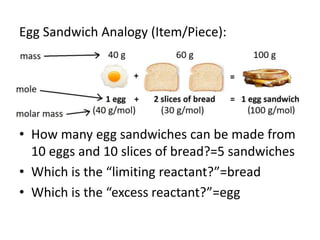
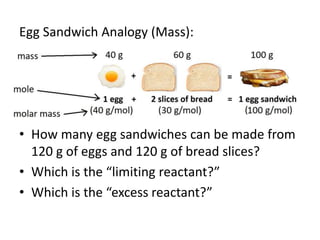
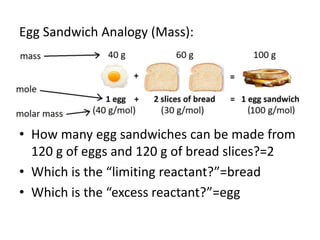
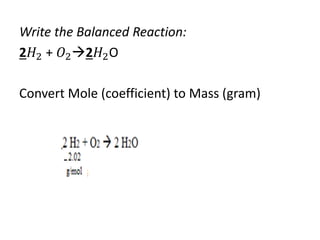
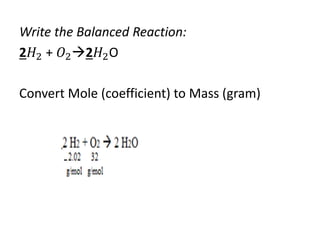
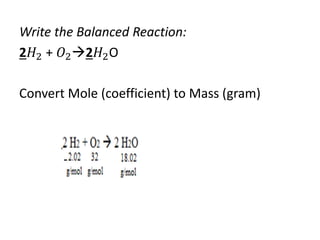
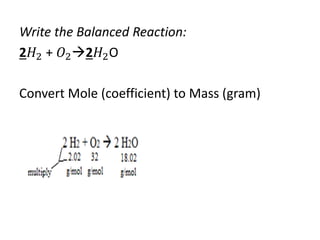
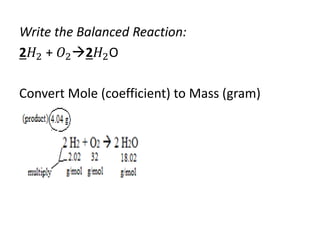
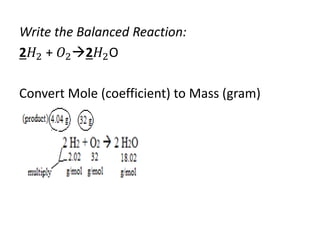
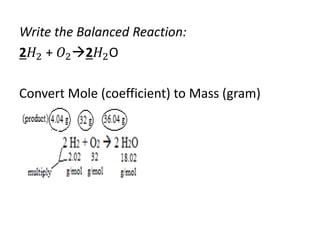
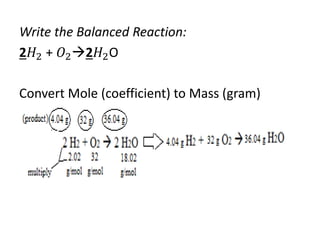
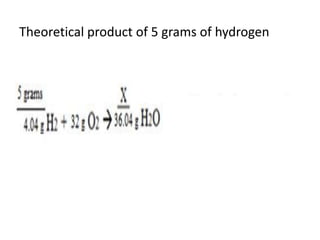
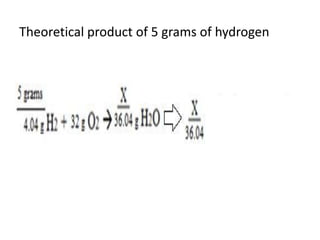
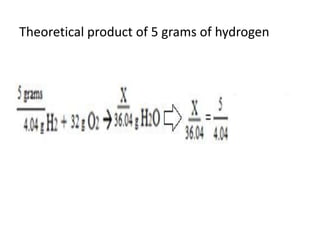
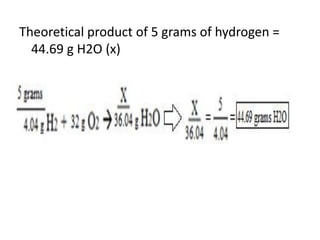
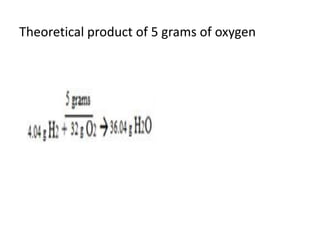
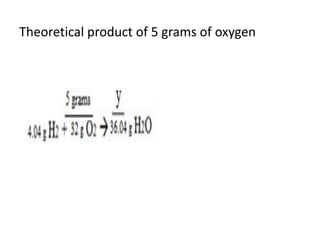
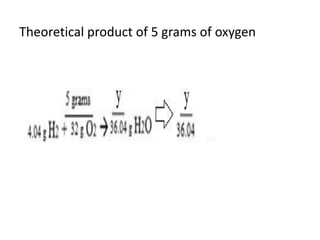
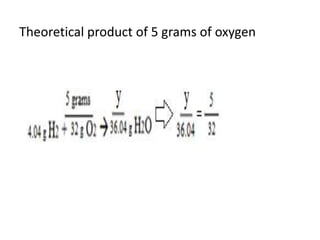
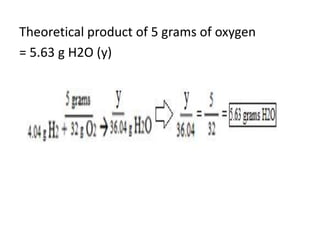
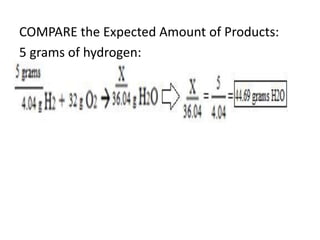
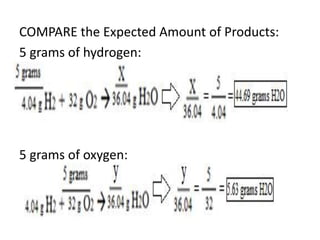
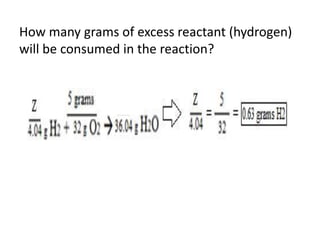
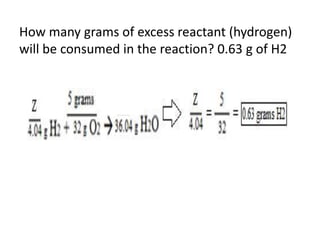
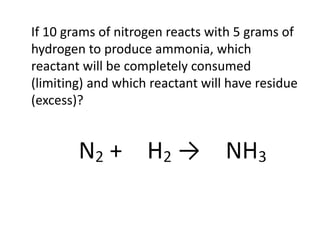
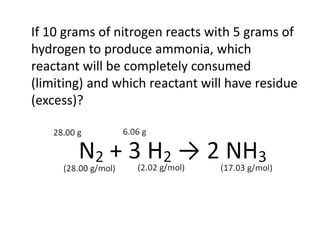
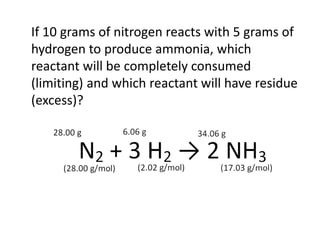
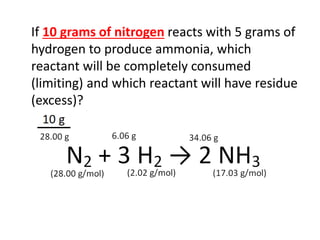
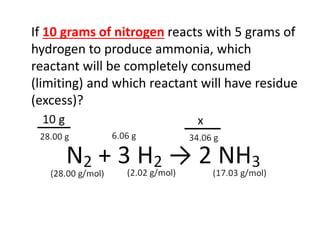
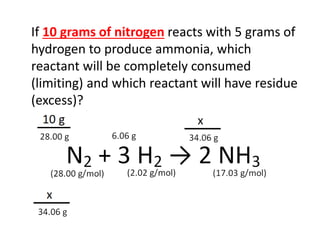
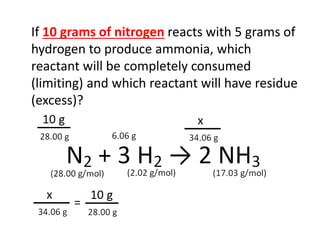
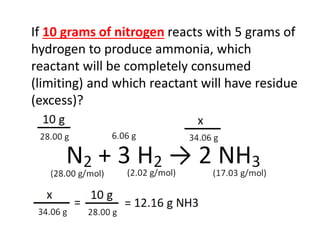
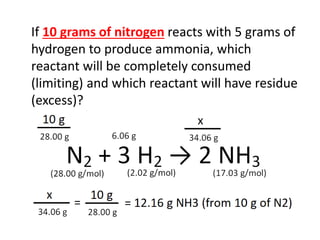
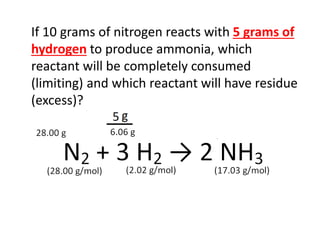
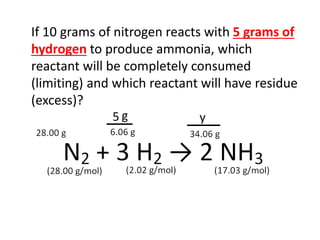
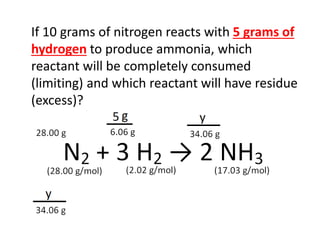
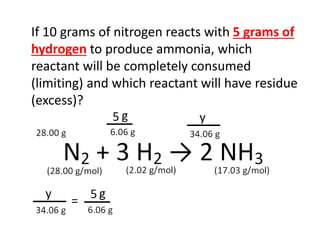
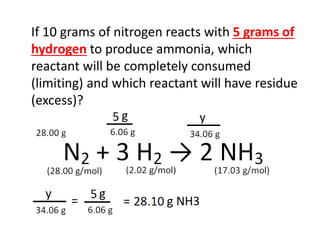
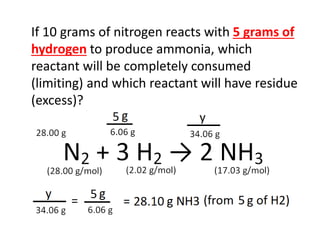
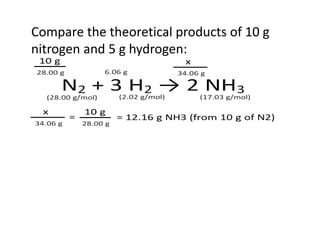
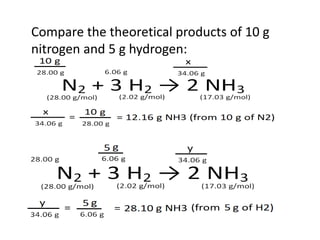
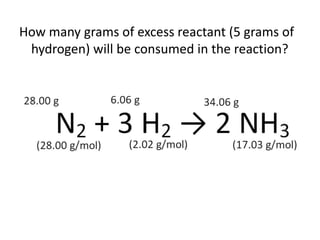
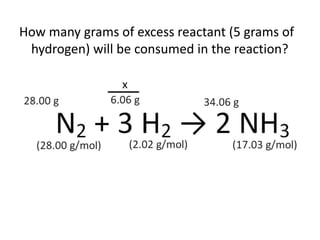
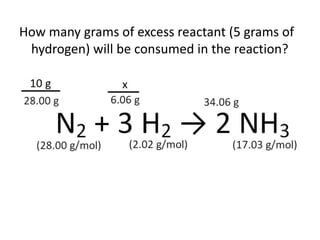
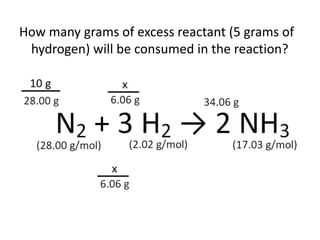
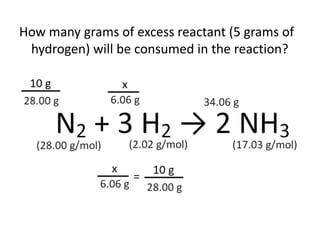
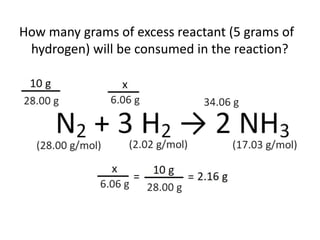
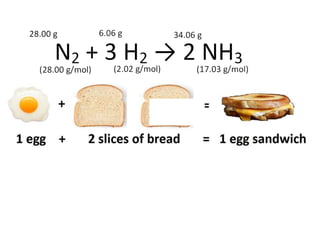
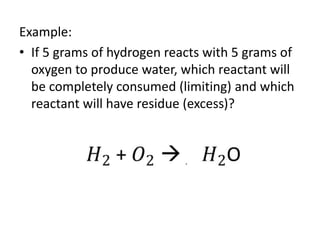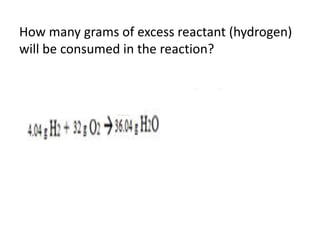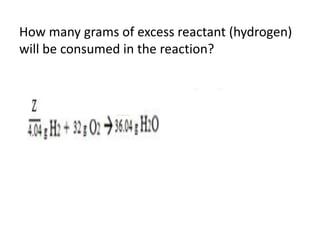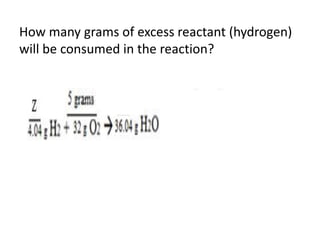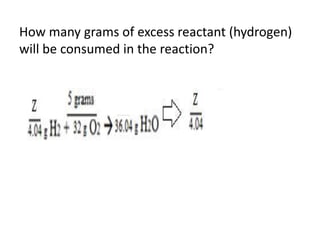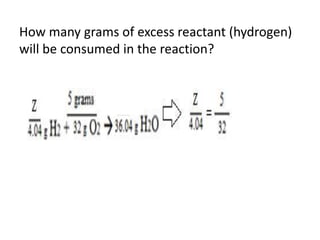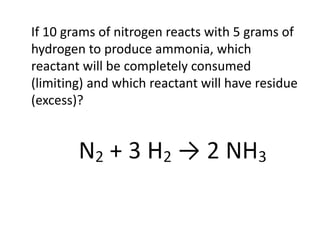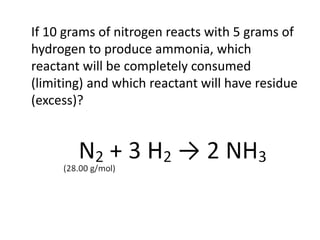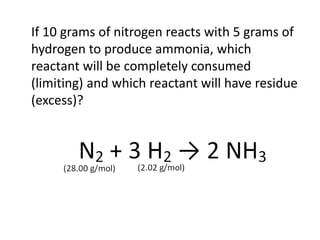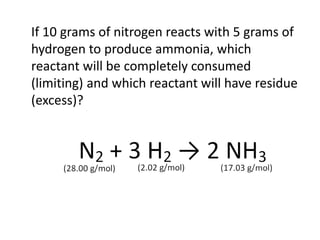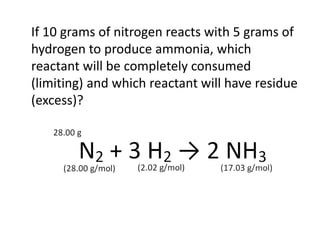The document discusses limiting and excess reactants using egg sandwich and chemical reaction analogies. It explains that the limiting reactant is completely consumed during a reaction and produces the lesser amount of product, while the excess reactant is not fully used and can produce more product. Examples then determine which reactant is limiting and which is excess based on theoretical product calculations when given mass amounts of reactants.