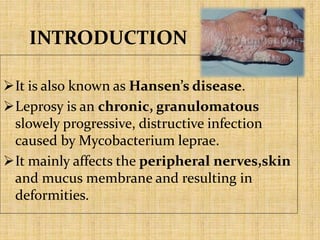
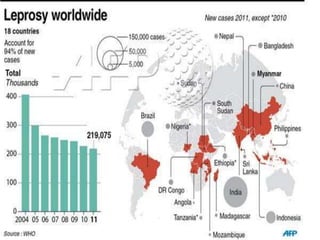
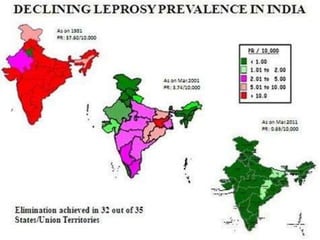
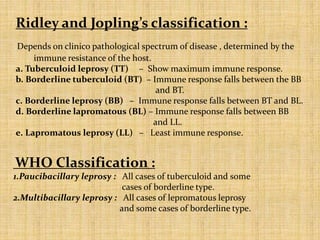
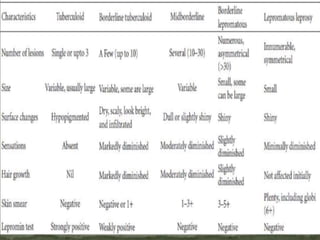
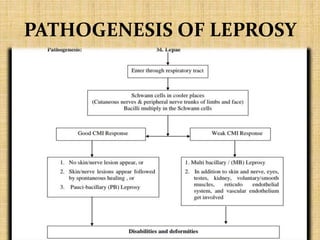
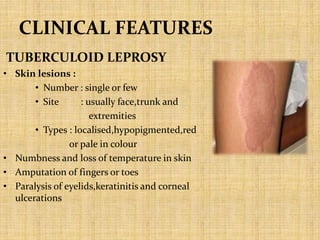
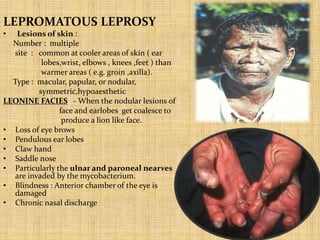
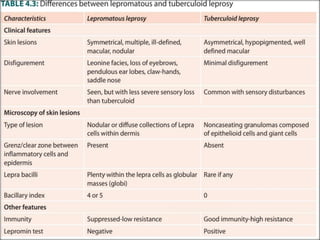
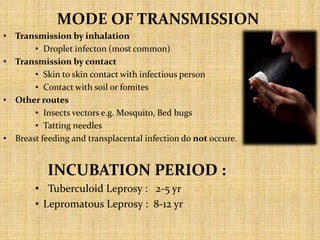
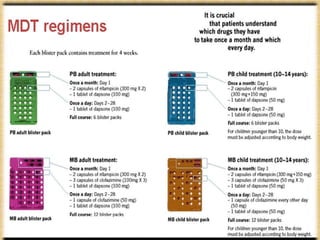
This document provides an overview of leprosy (Hansen's disease). It discusses the history, epidemiology, etiology, classification, pathogenesis, transmission, clinical features, treatment, reactions, and management of leprosy. Leprosy is caused by Mycobacterium leprae and mainly affects the skin and peripheral nerves. It is treated using multidrug therapy regimens as recommended by the WHO. Management involves treatment, prevention of disabilities, and social integration programs.