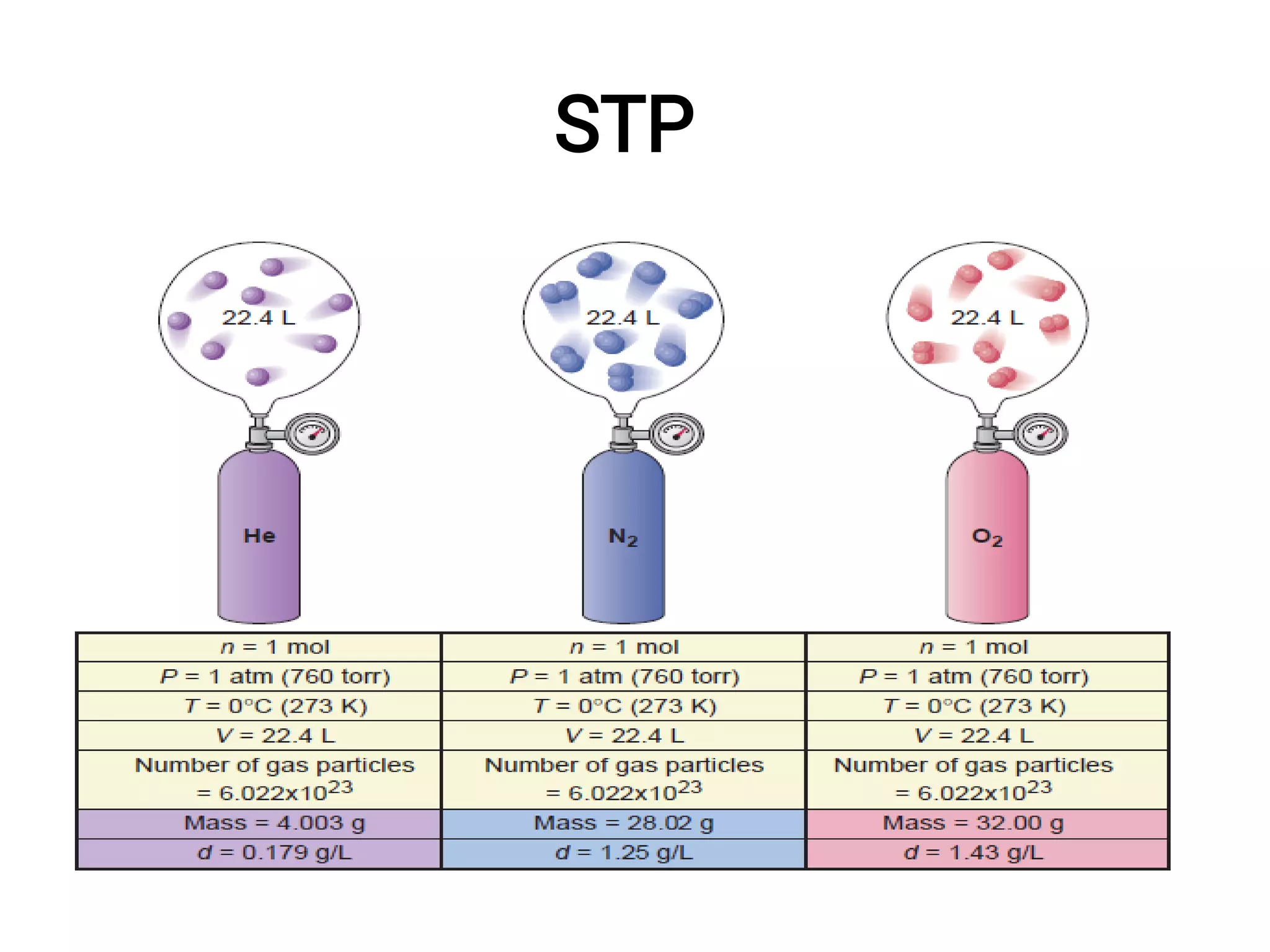
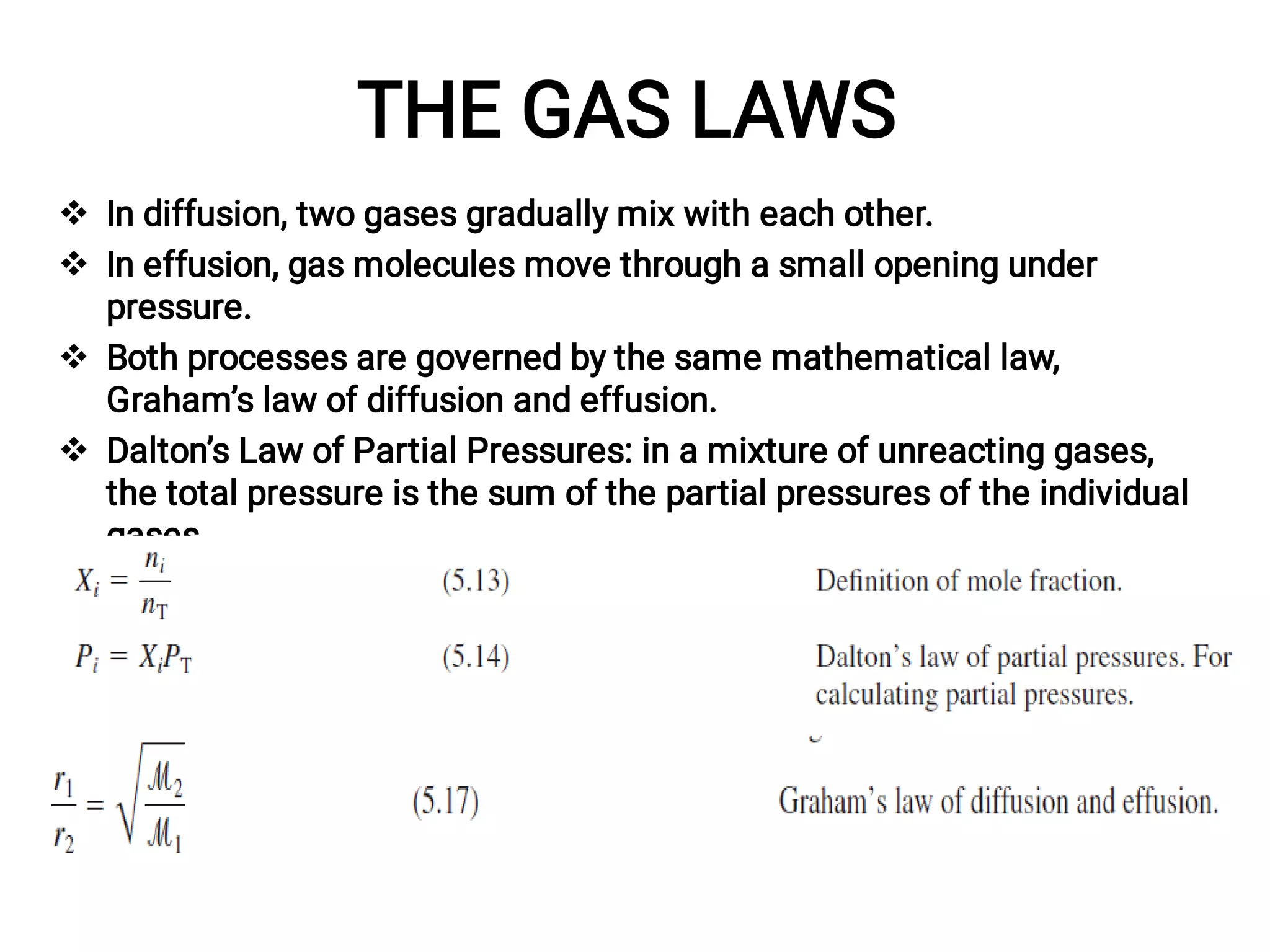
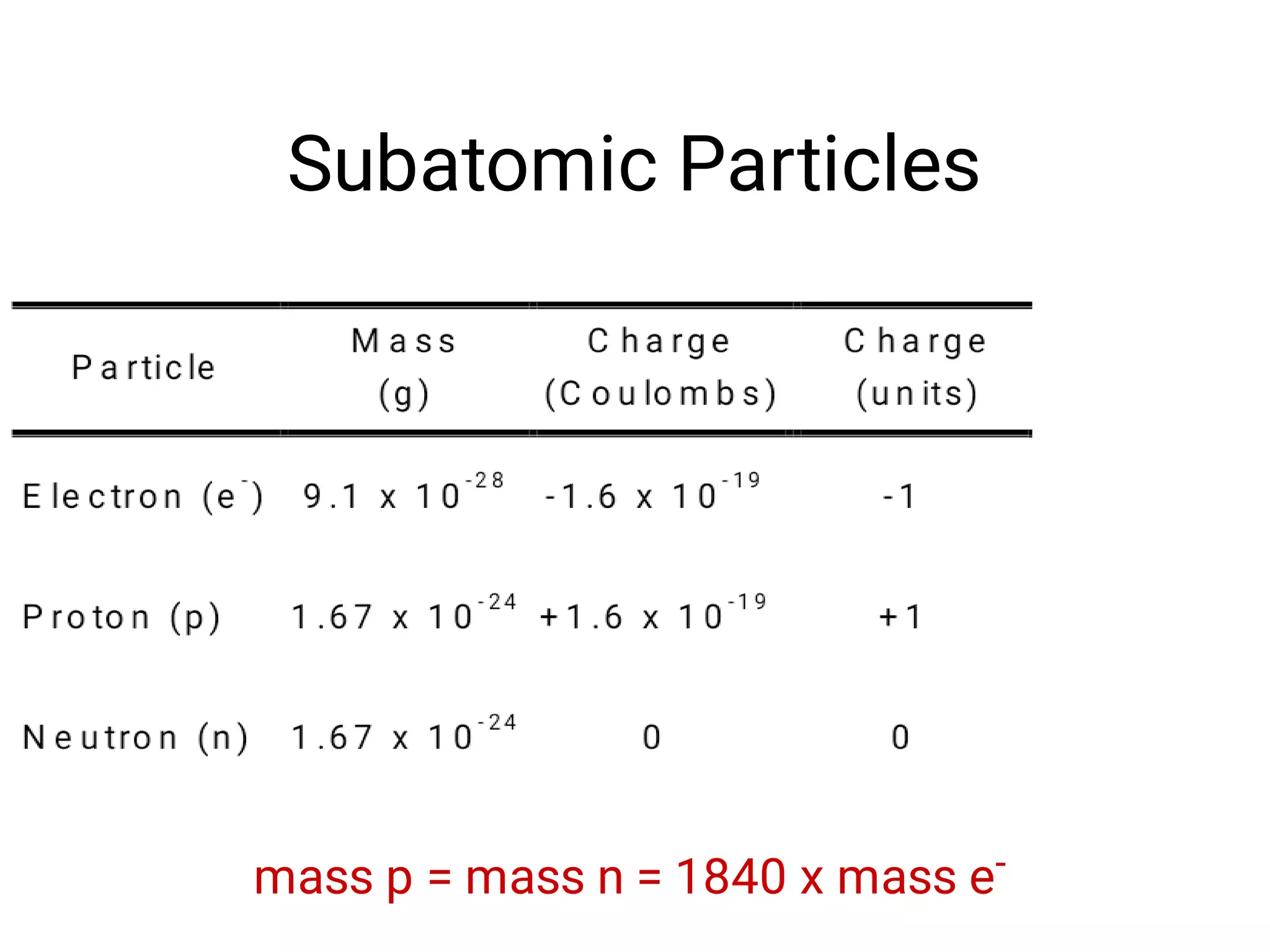
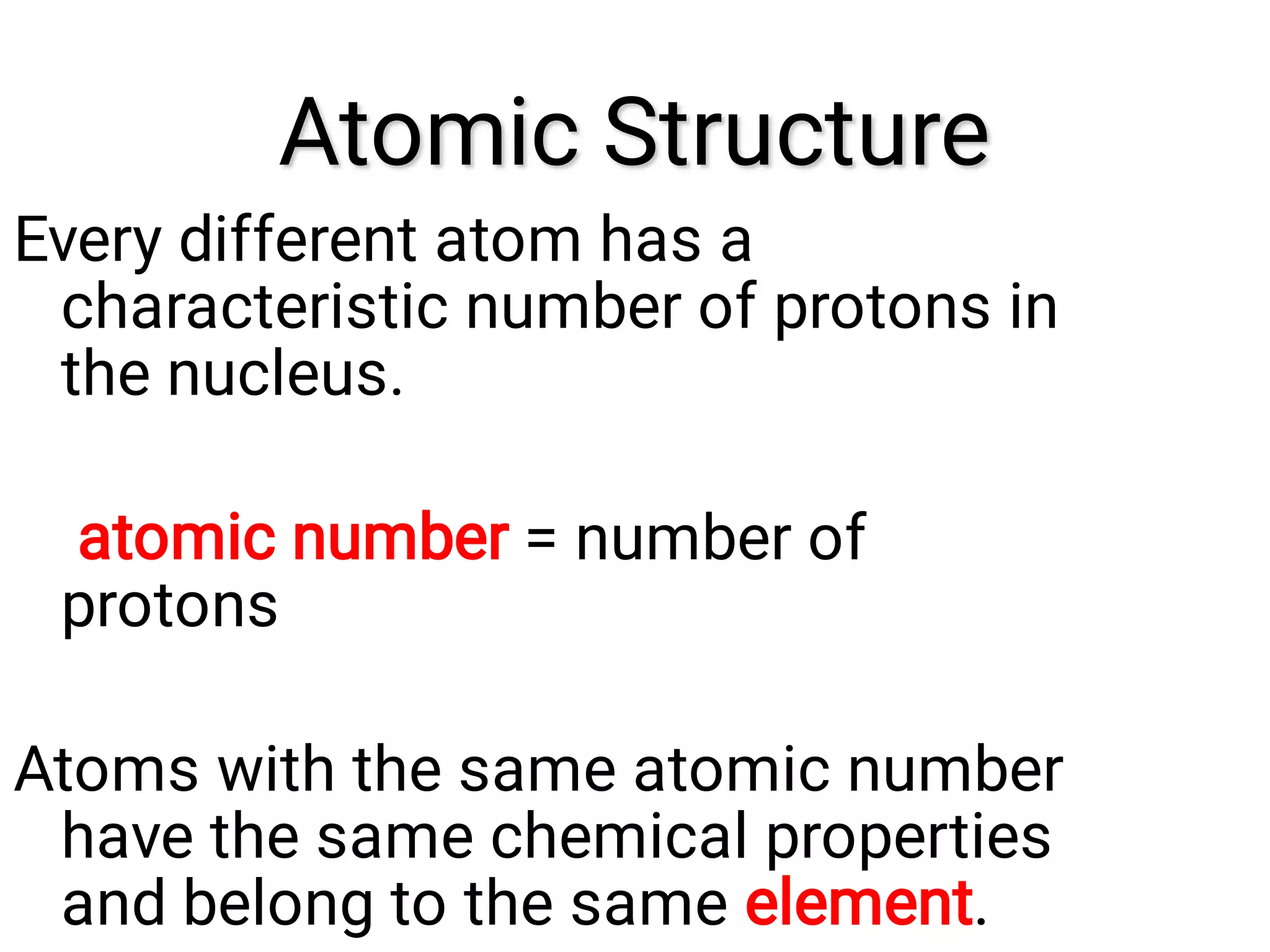
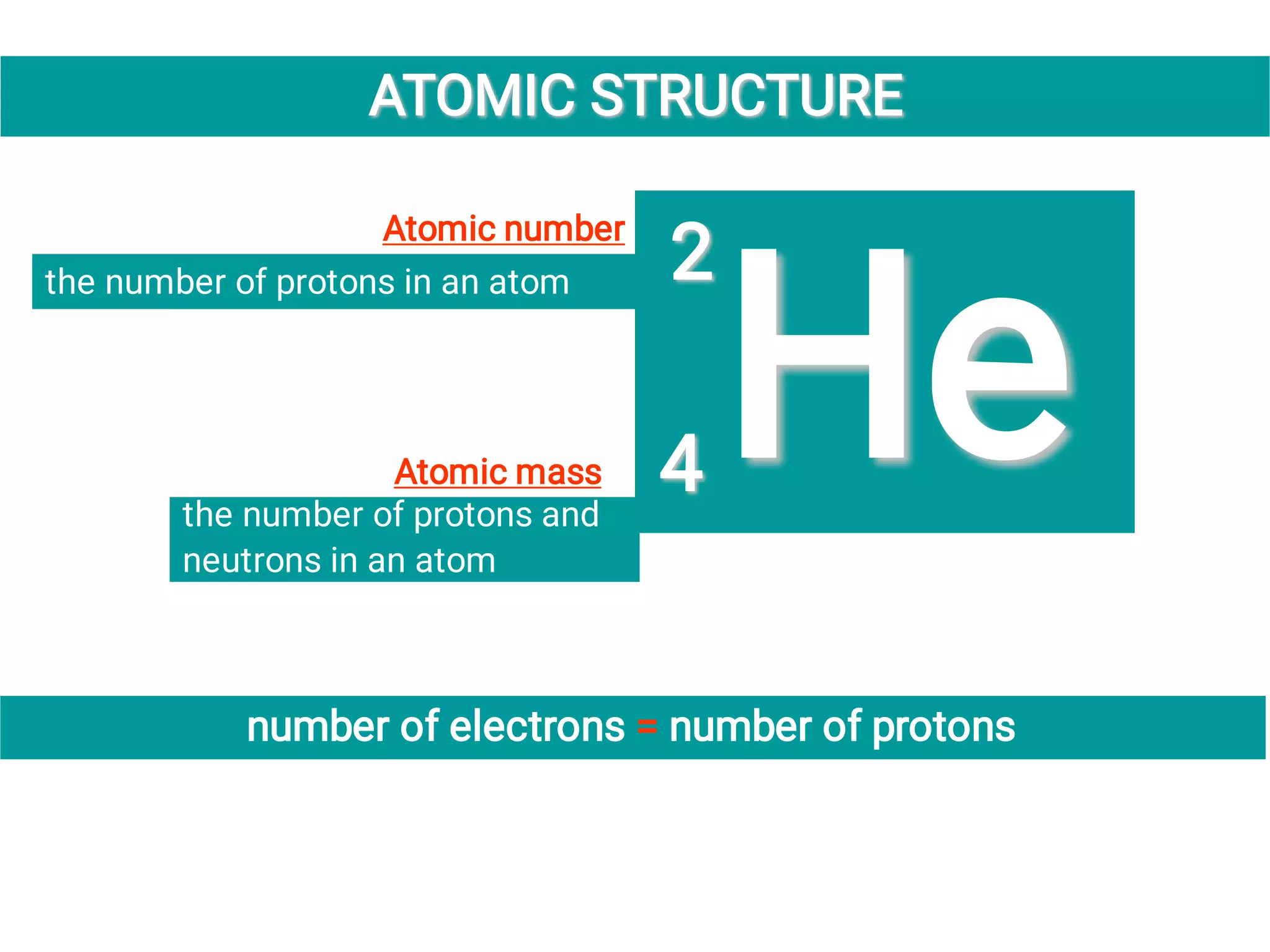
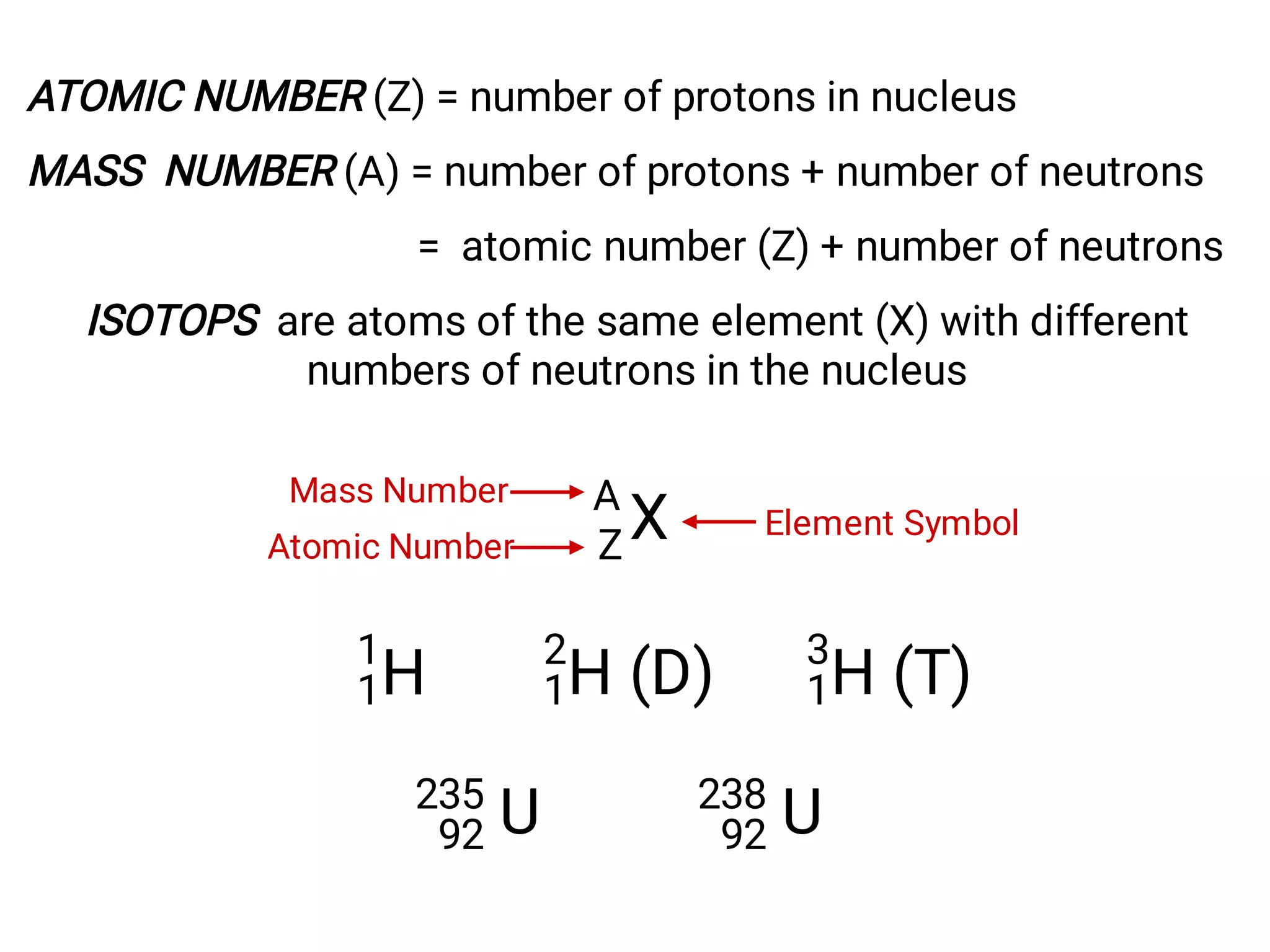
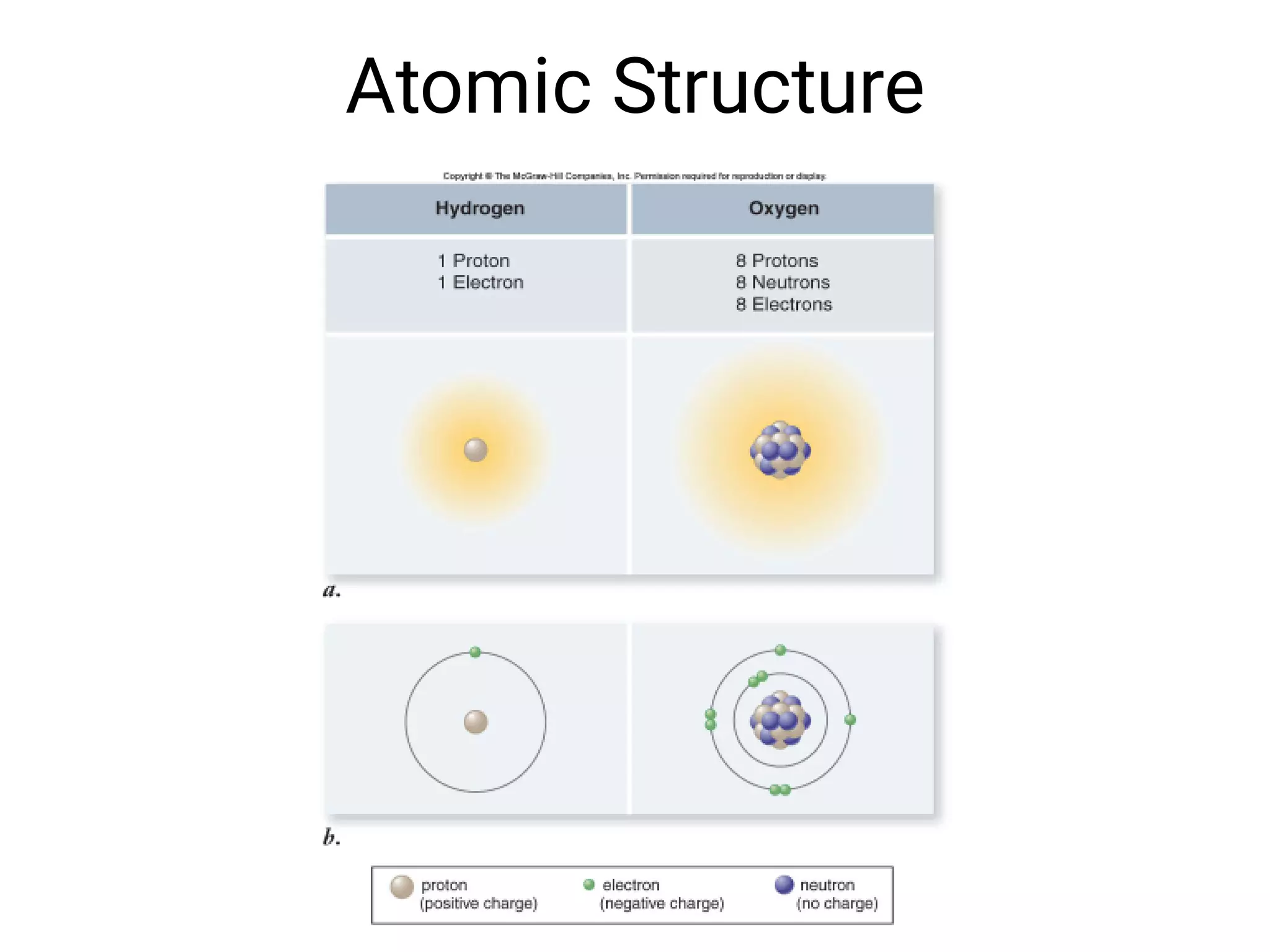
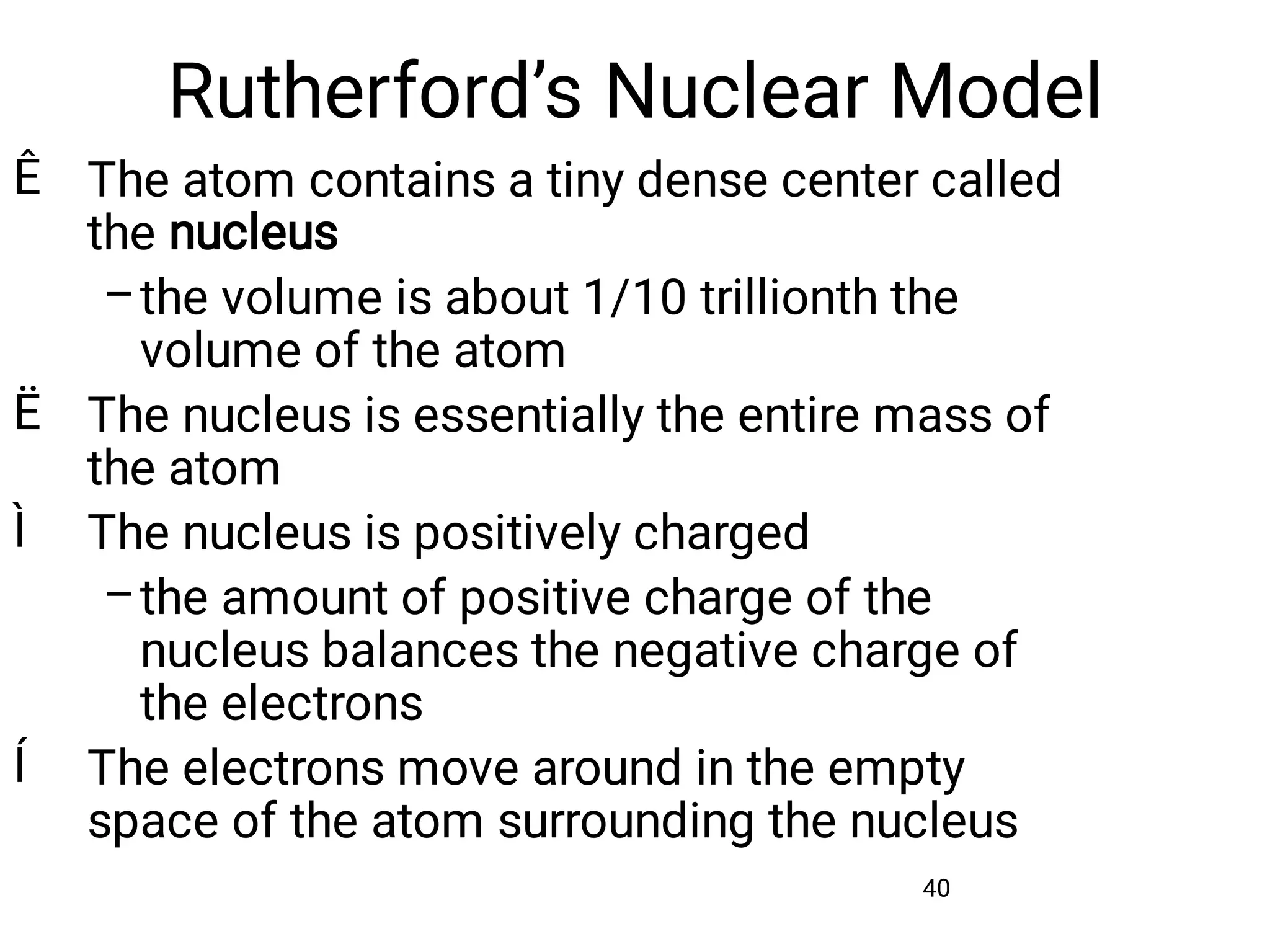
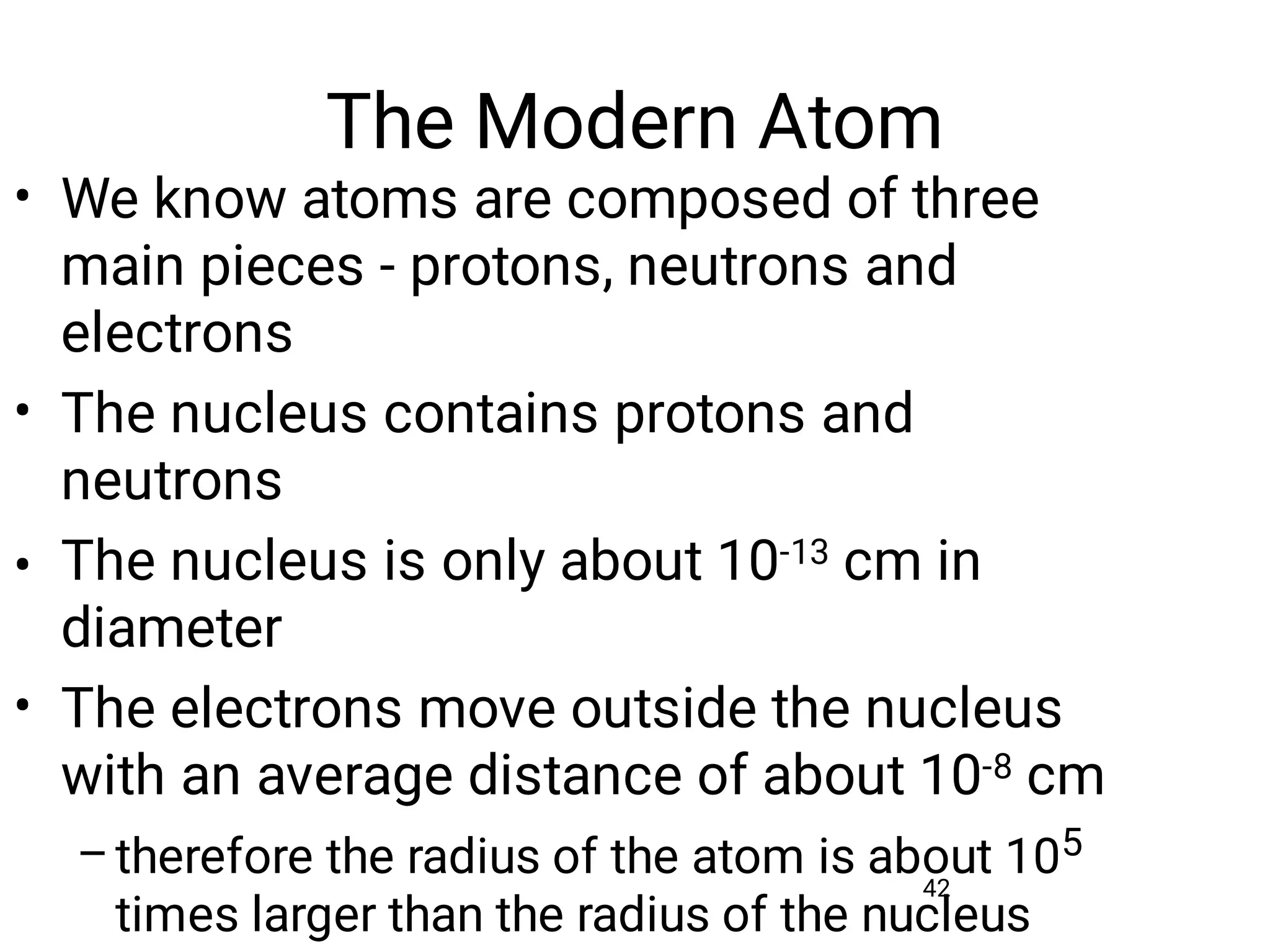
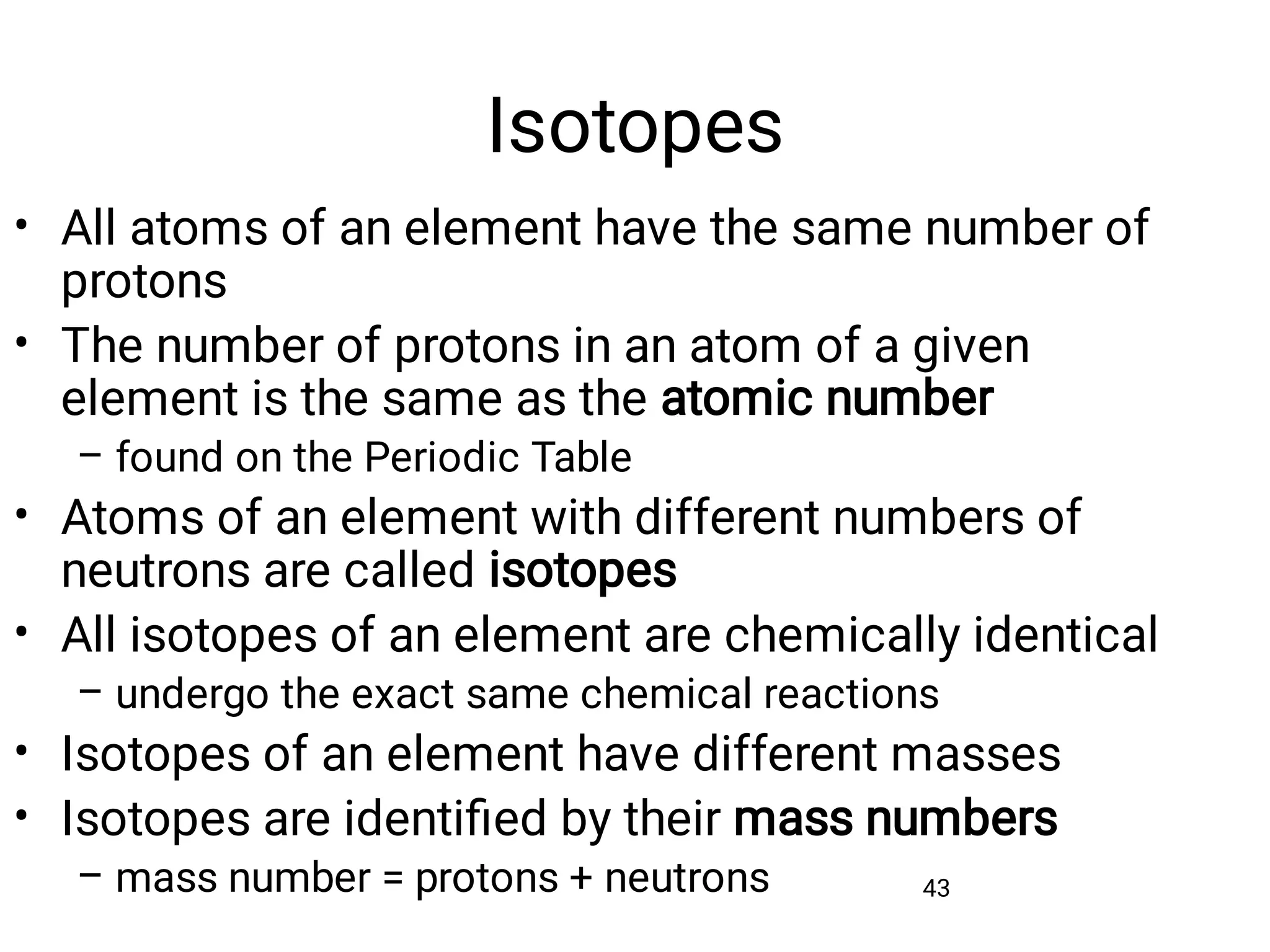
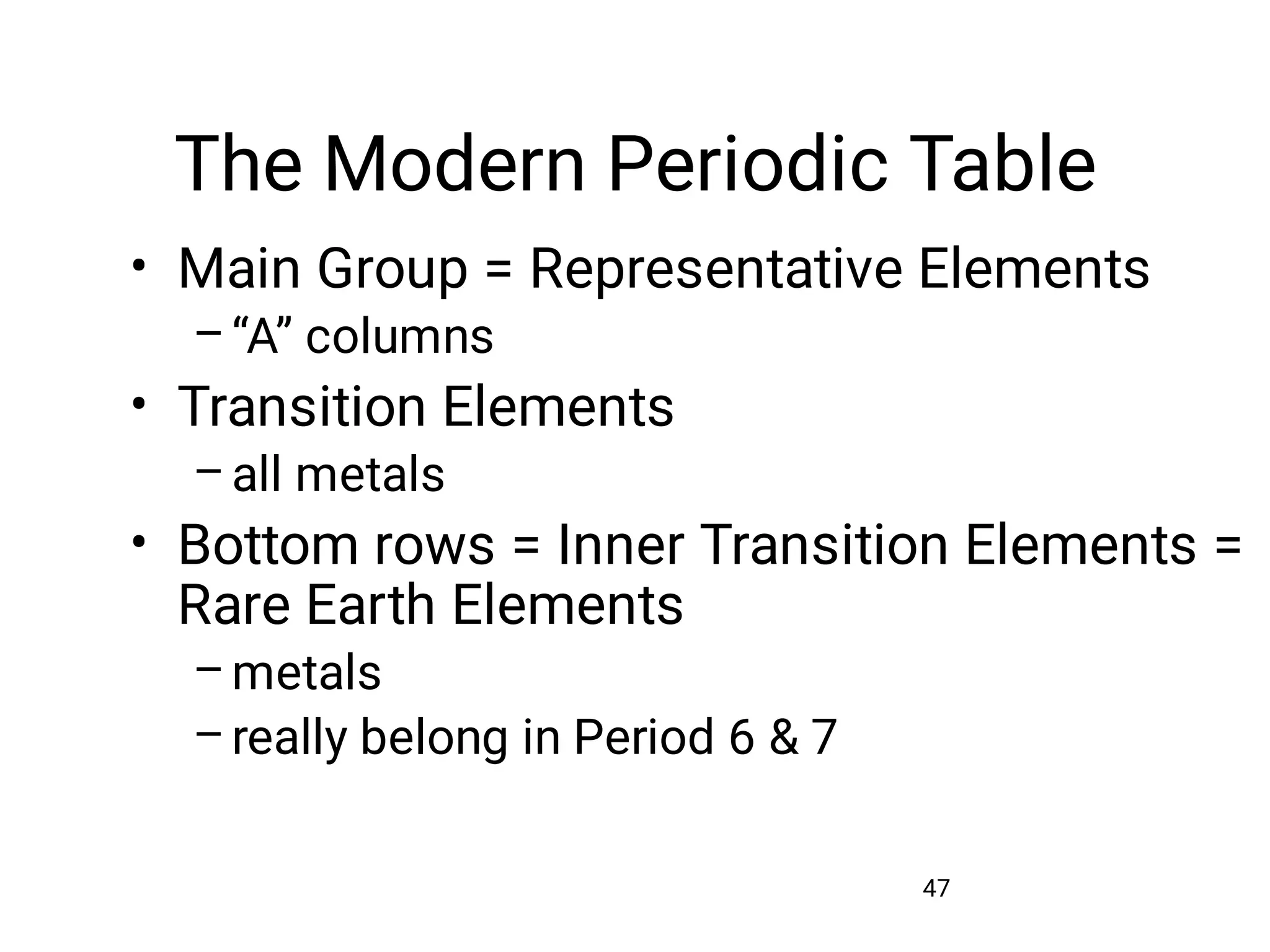
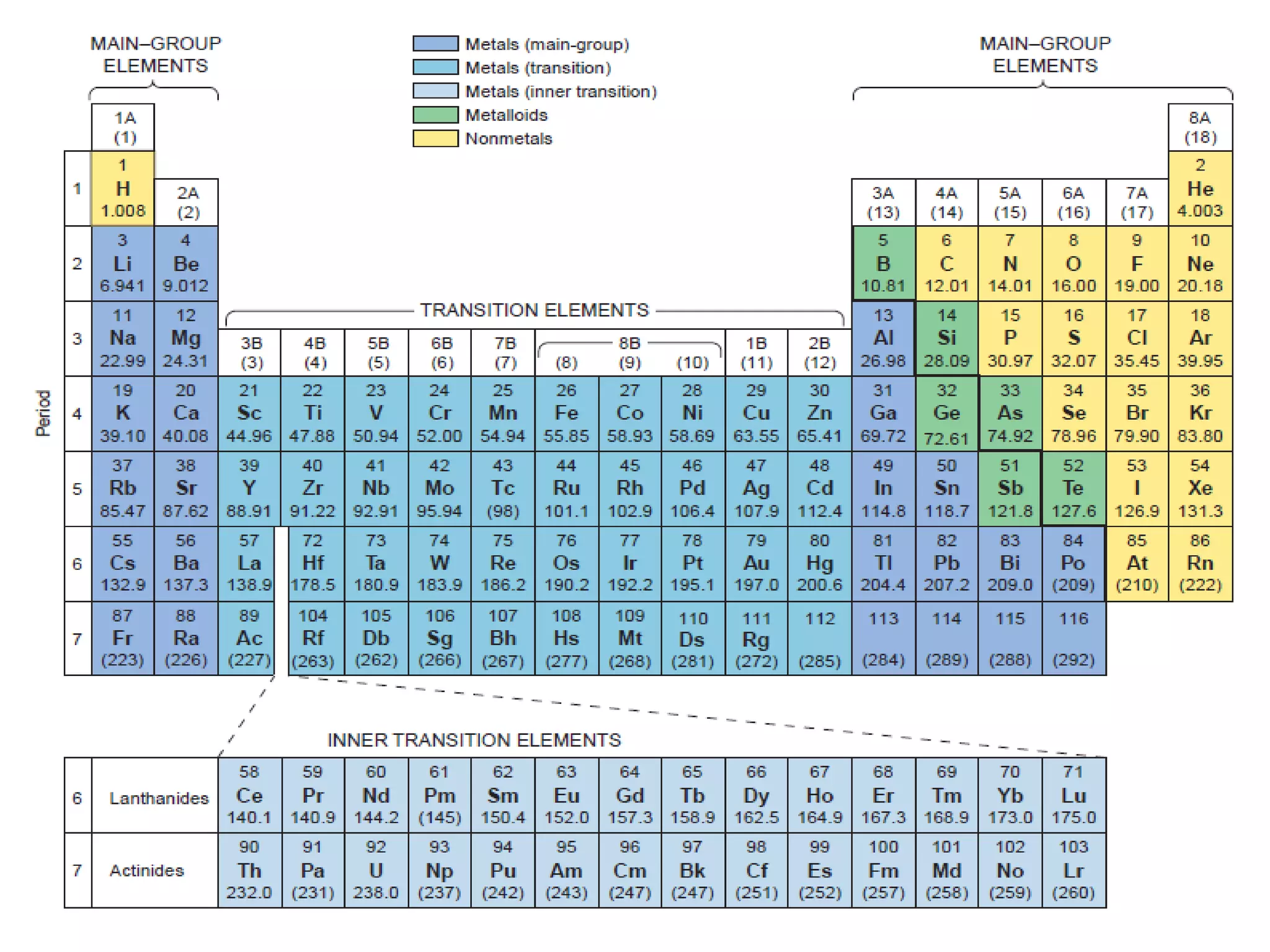
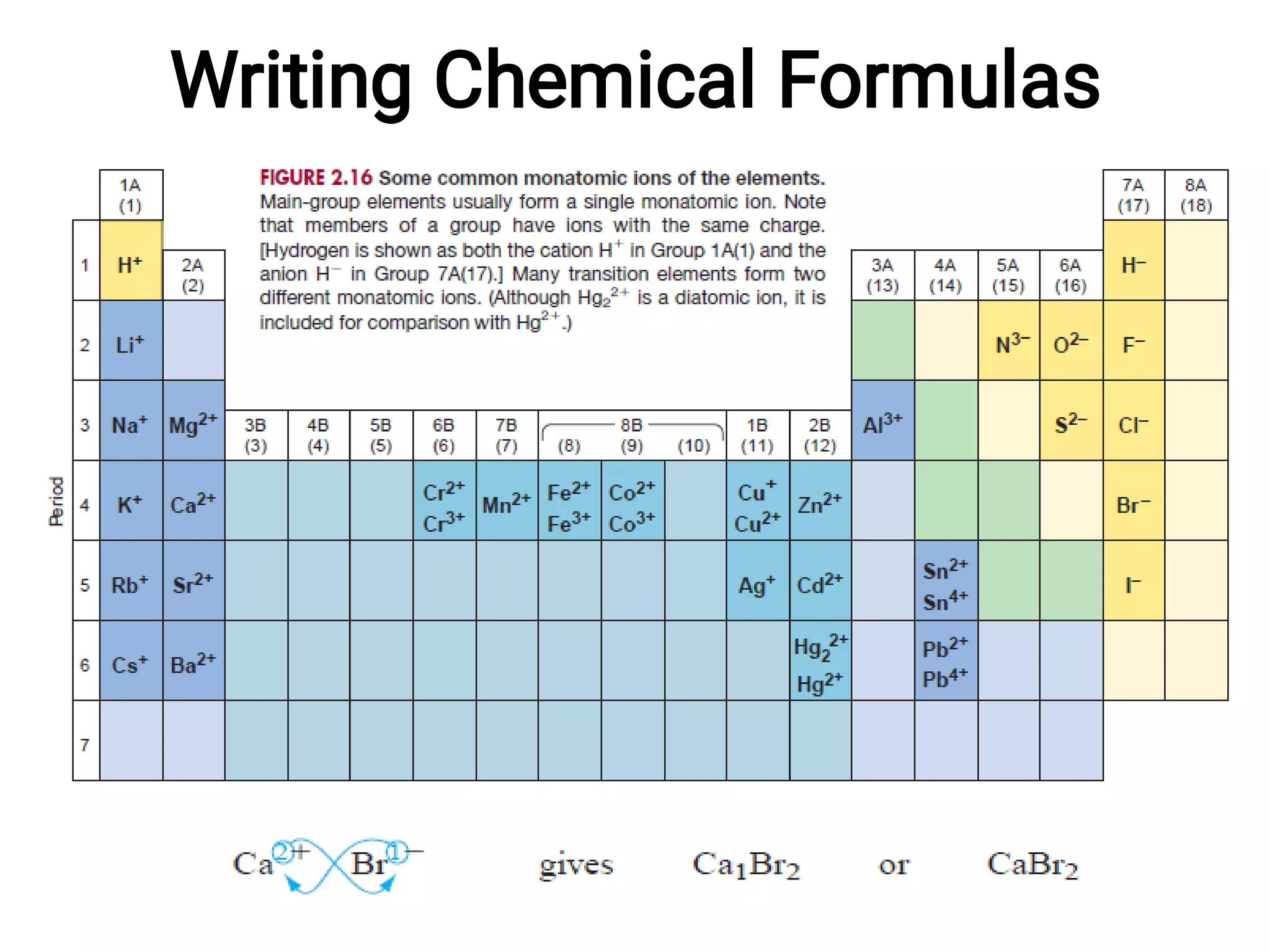
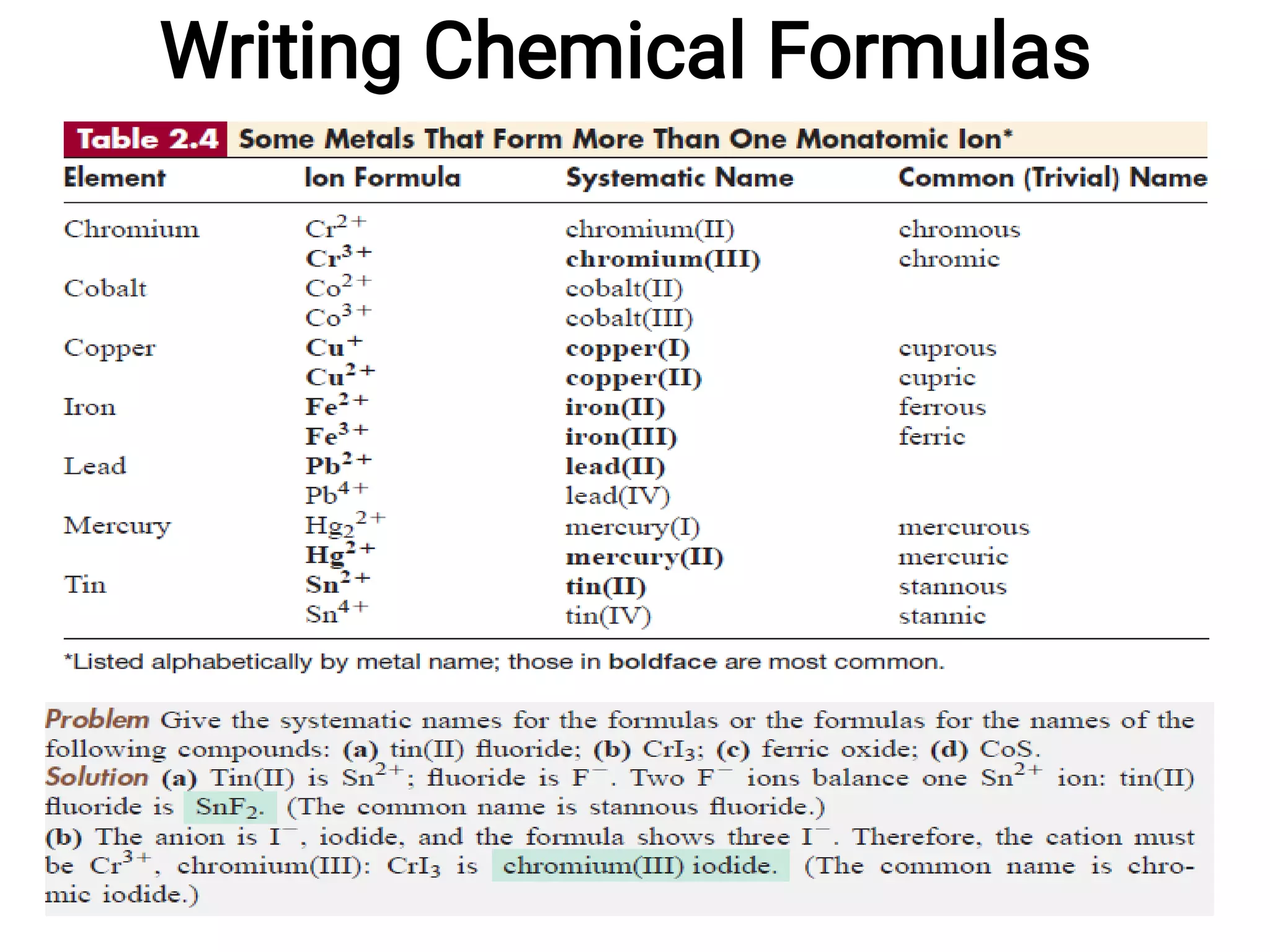
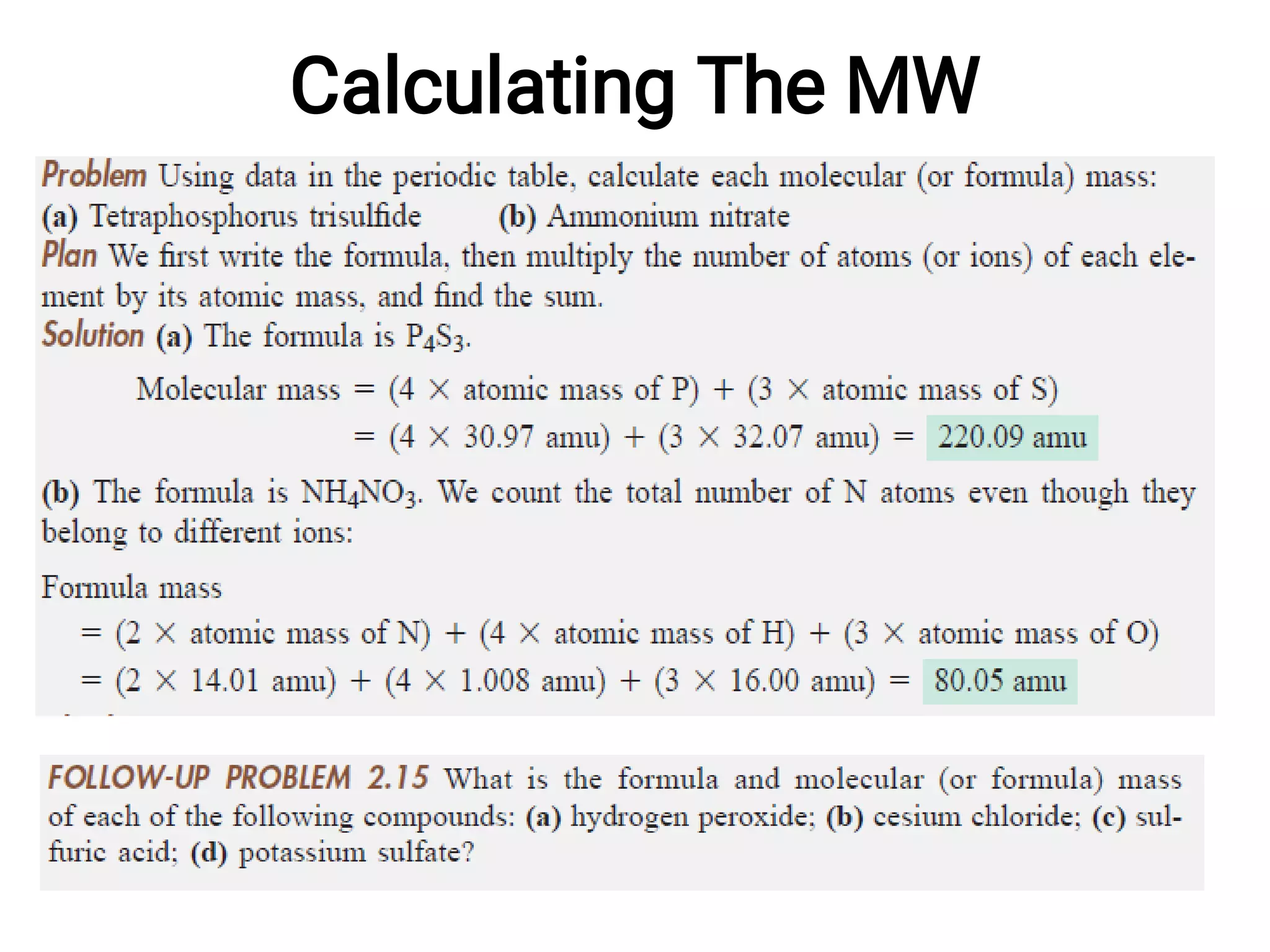
Mass and density are physical properties of matter. Mass is the amount of matter in an object, while density is the ratio of mass to volume. Temperature is a measure of how hot or cold an object is, and heat is the transfer of energy between objects at different temperatures. The three main temperature scales are Celsius, Kelvin, and Fahrenheit. Atoms are the basic building blocks of matter and are composed of protons, neutrons, and electrons. The atomic structure and arrangement of subatomic particles determine an element's properties. Elements can form compounds by chemically combining in fixed ratios determined by their atomic structure.
























































![Quantum Theory and Atomic Structure
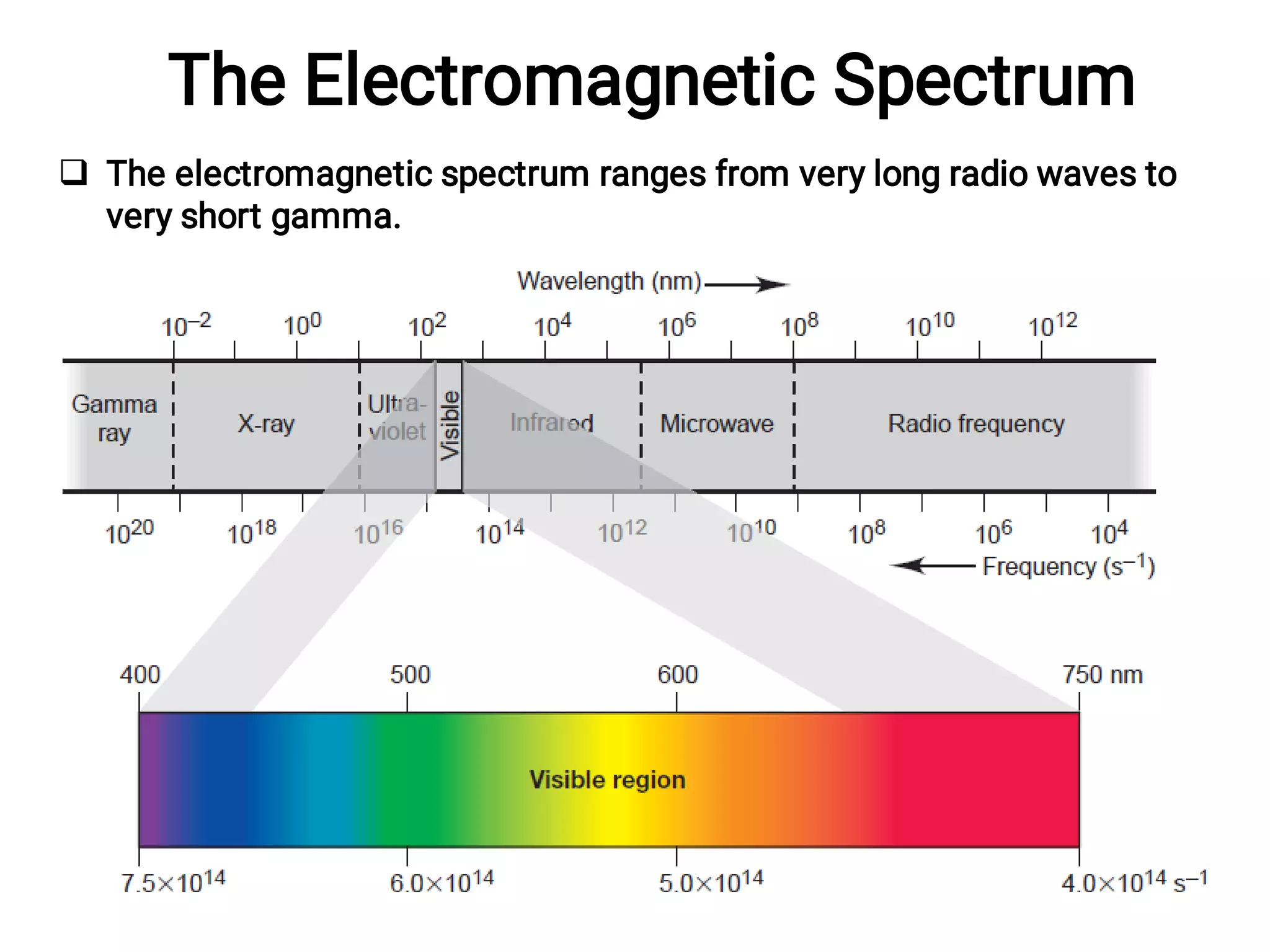
The Nature of Light
Electromagnetic radiation travels in waves of specific wavelength (λ)
and frequency (ν).
Frequency (nu, ν) is the number of cycles the wave undergoes per
second, expressed in 1/second [s-1; also called hertz (Hz)].
Wavelength (lambda, λ) is the distance between any point on a wave
and the corresponding point on the next crest, expressed in
nanometers (nm, 10-9 m).
Amplitude, the height of the crest (or depth of the trough) of each
wave.
All electromagnetic waves travel through a vacuum at the speed of
light, c (3.00X108 m/s).
Radiation with a high frequency has a short wavelength, and vice
versa.](https://image.slidesharecdn.com/45778236631085485478-230115122223-1a405cbd/75/Basis-for-chemistry-72-2048.jpg)









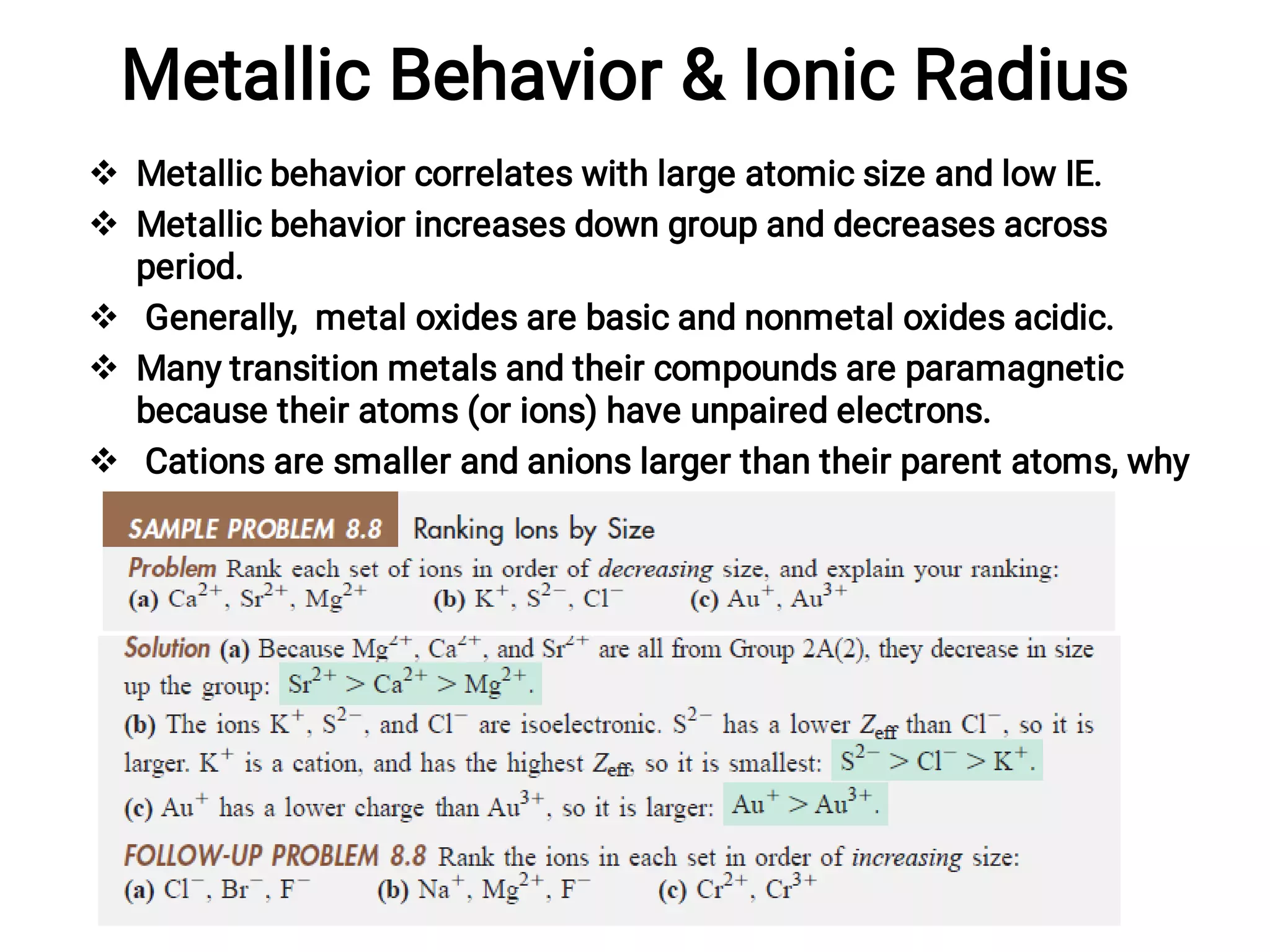










![pH Scale
Pure water has low conductivity because it autoionizes to small
extent.
Ionization constant for water, Kw (1.0 x10-14 at 25°C).
in acidic solution, [H3O+] is greater than [OH]; the reverse is true in
basic solution; and the two are equal in neutral solution.
Simply, we use the pH scale (pH = -log [H3O+]).](https://image.slidesharecdn.com/45778236631085485478-230115122223-1a405cbd/75/Basis-for-chemistry-96-2048.jpg)