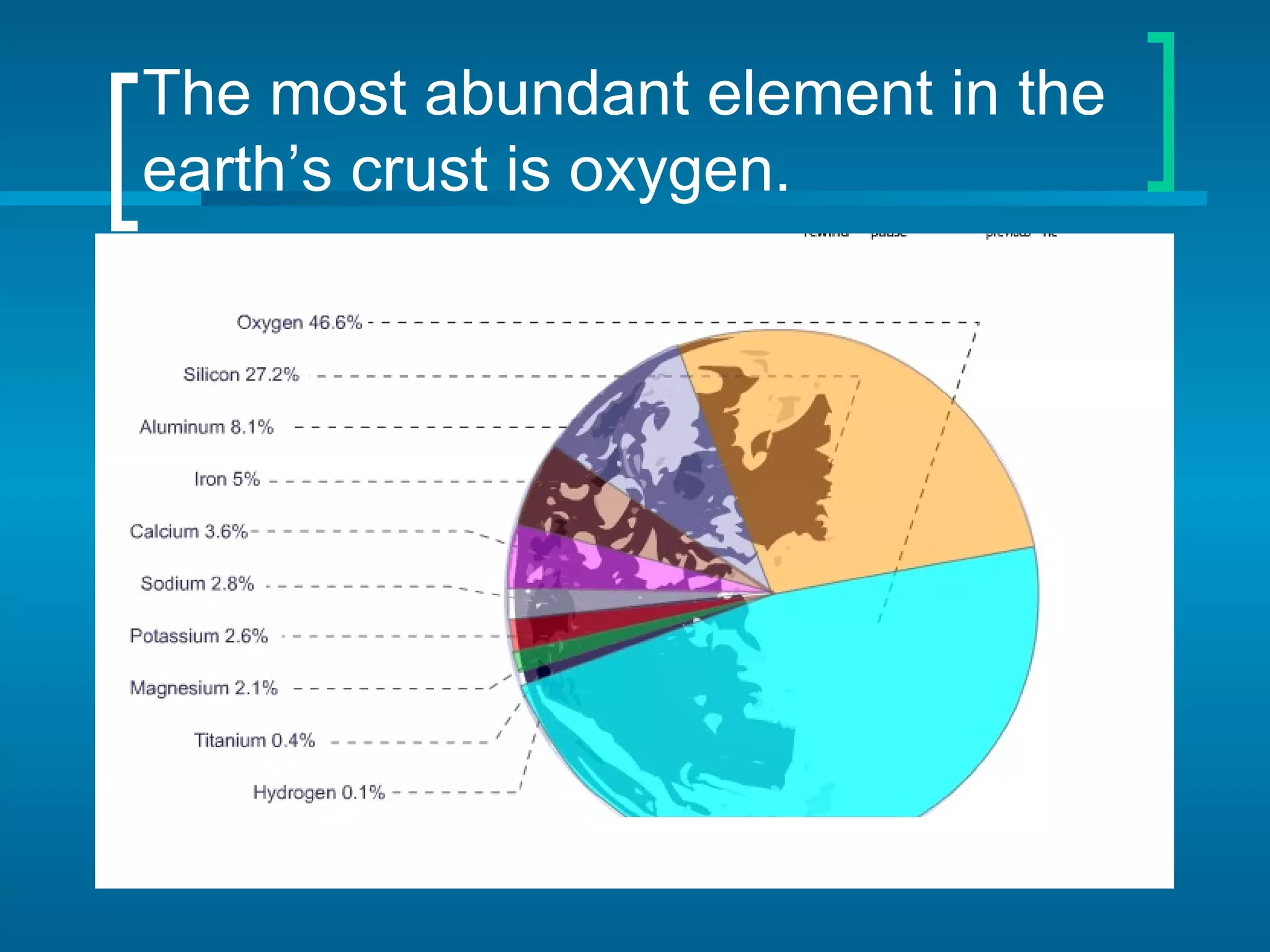
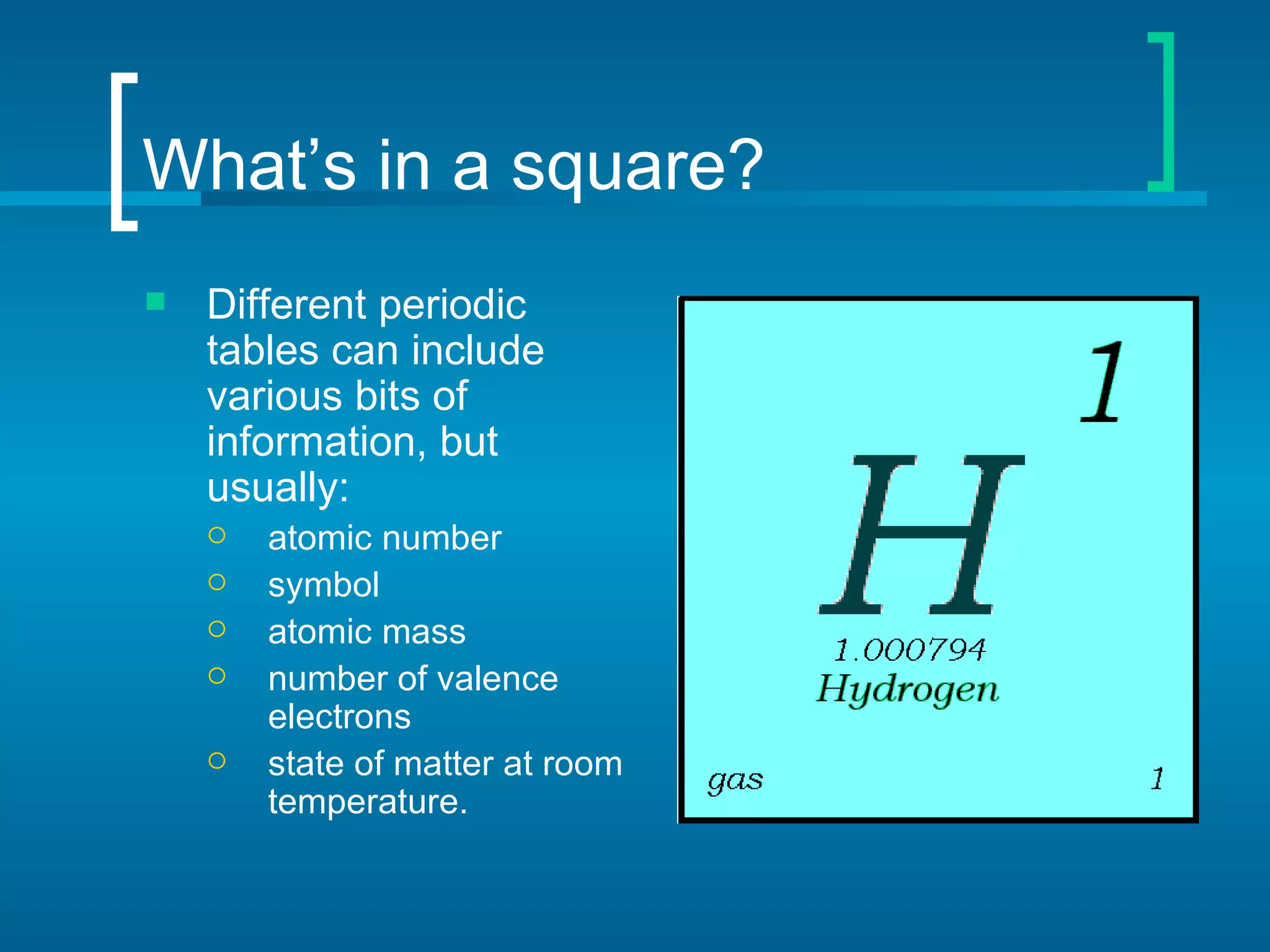
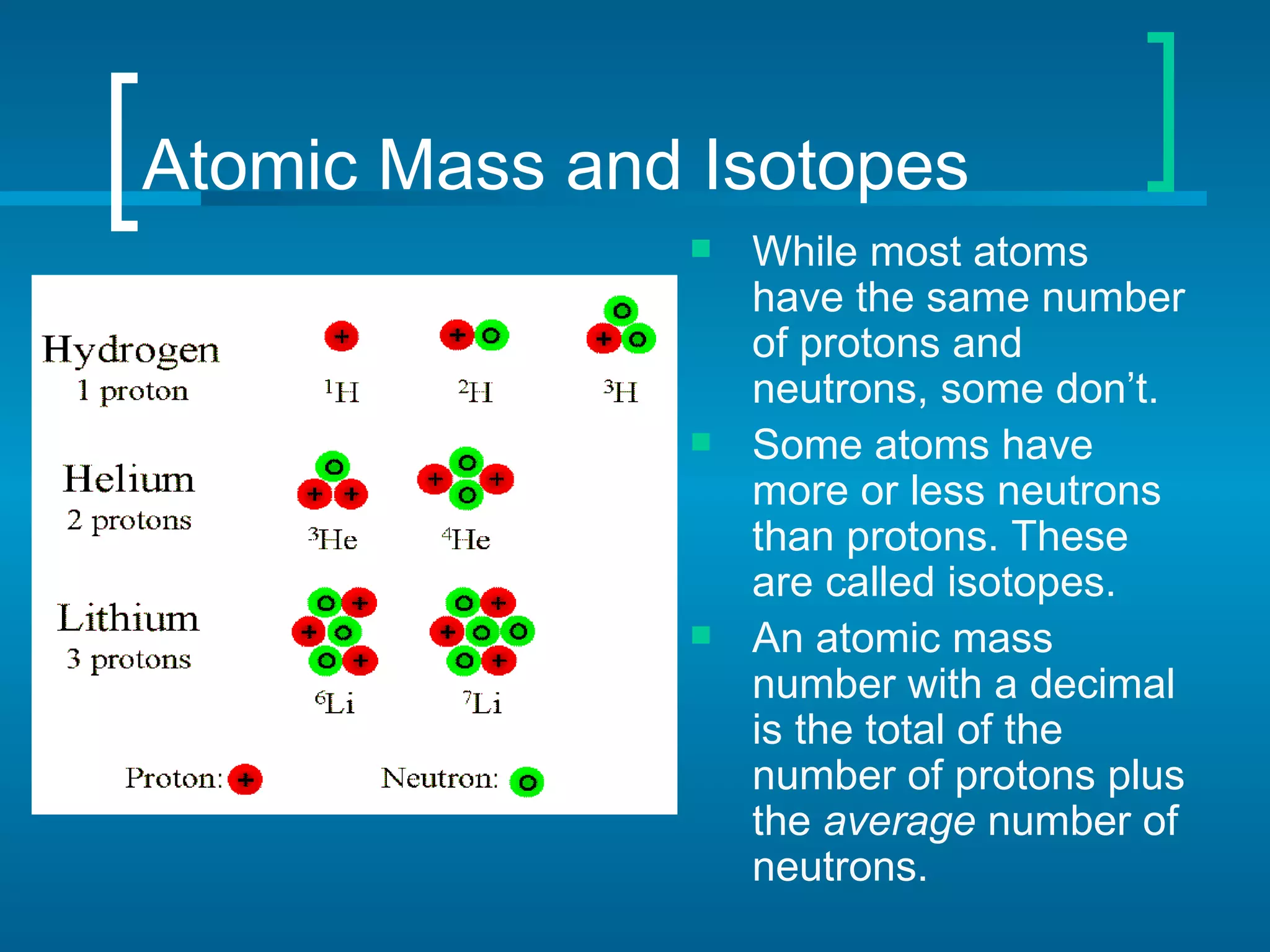
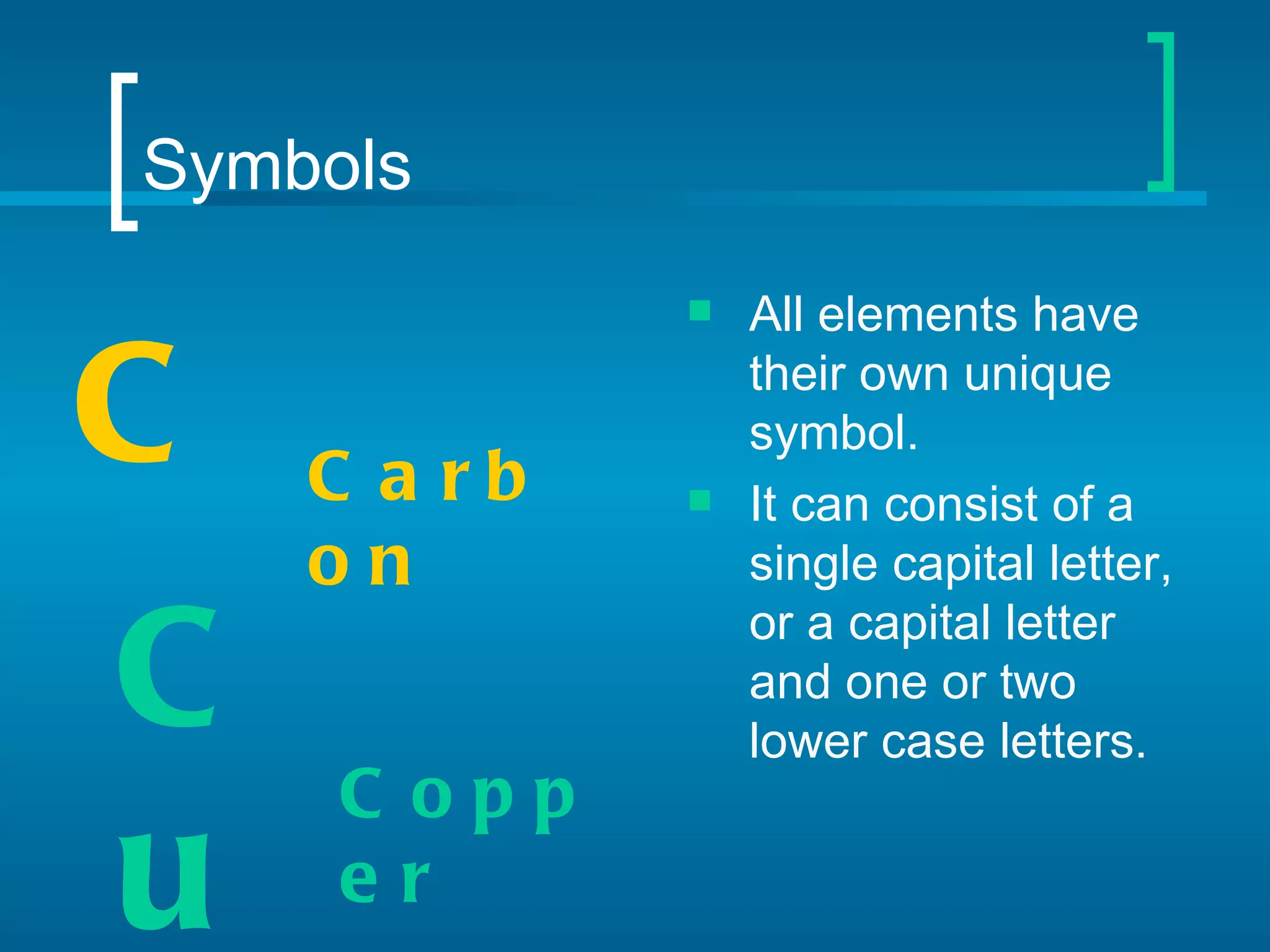
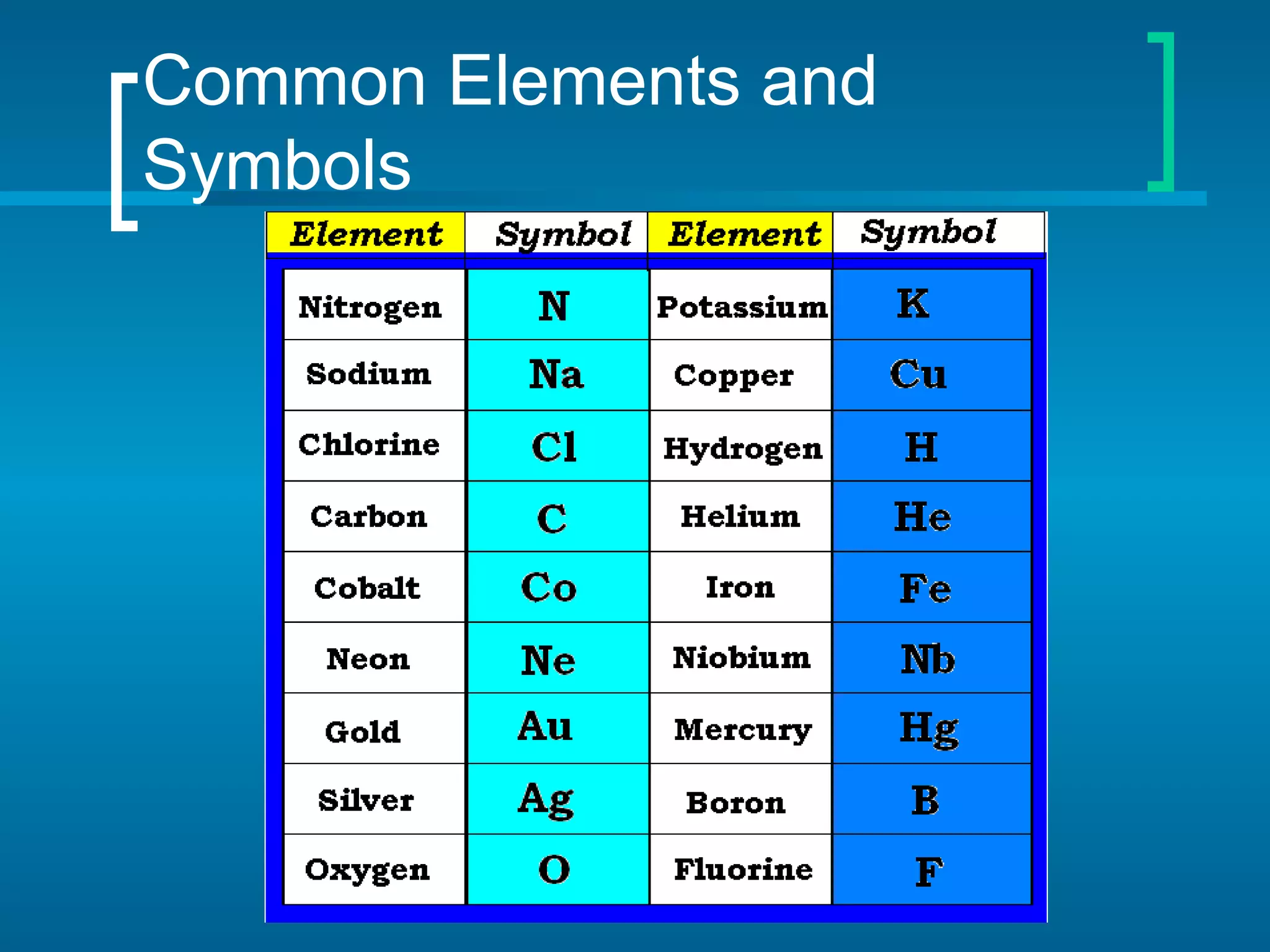
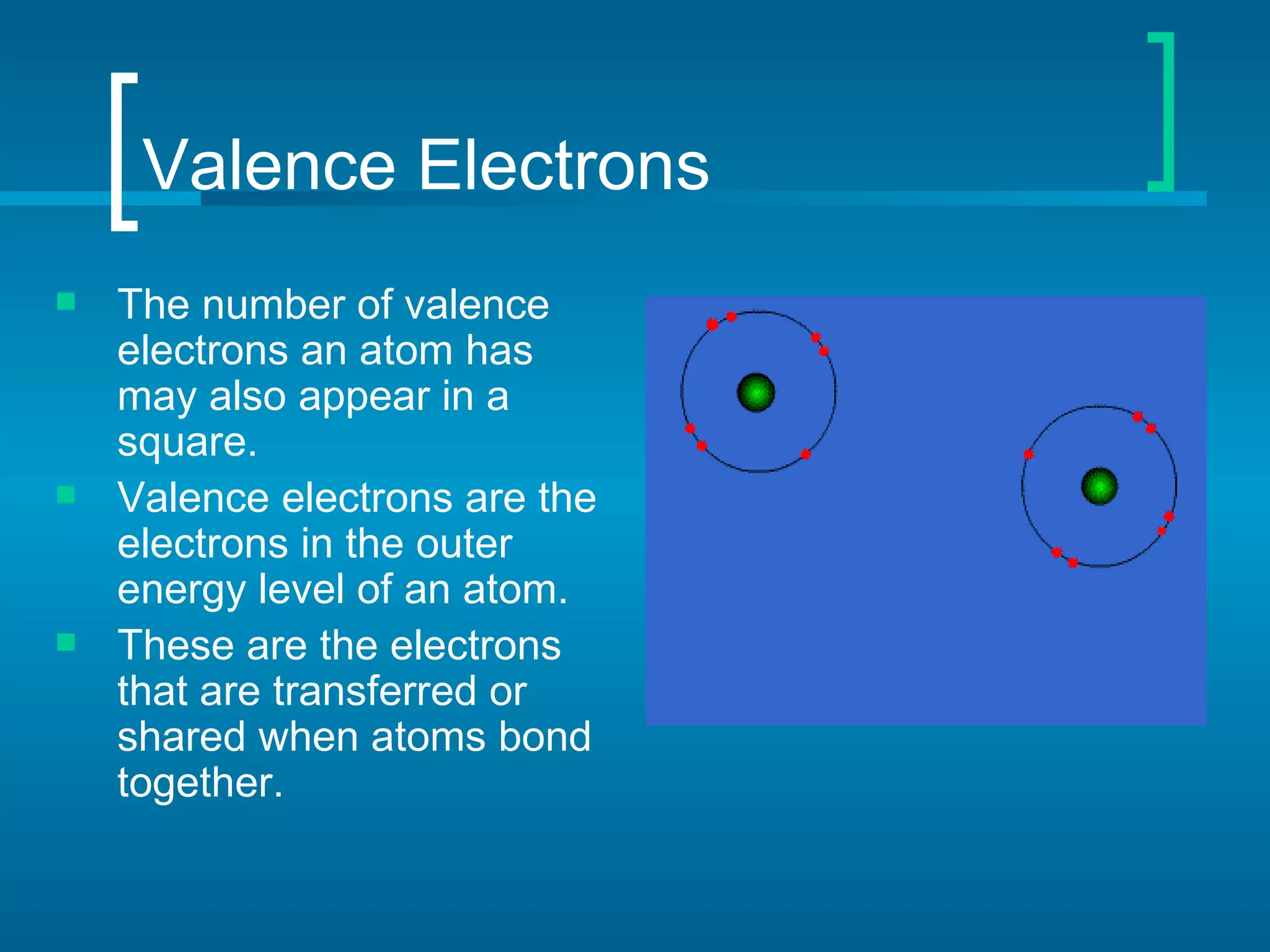
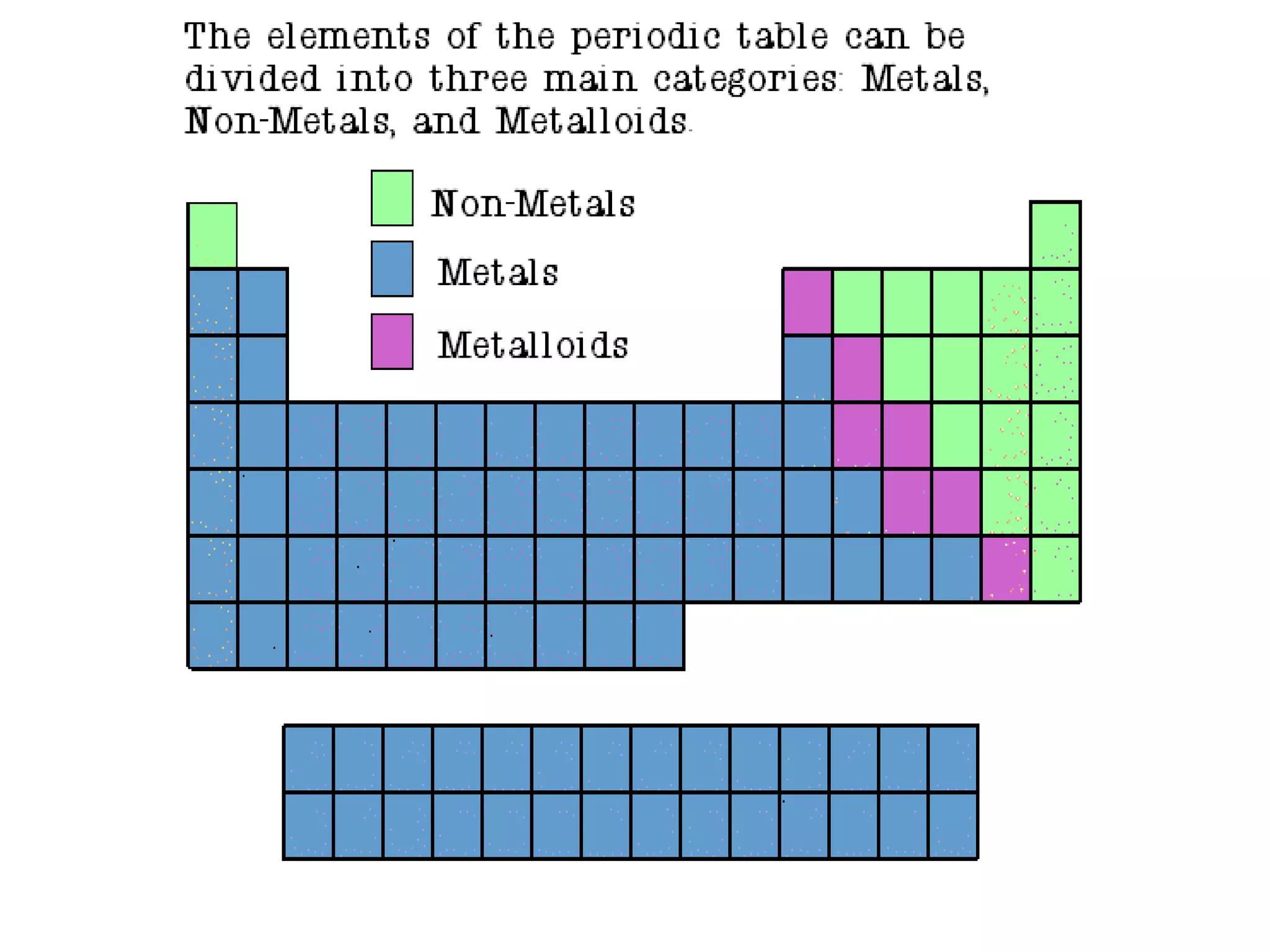
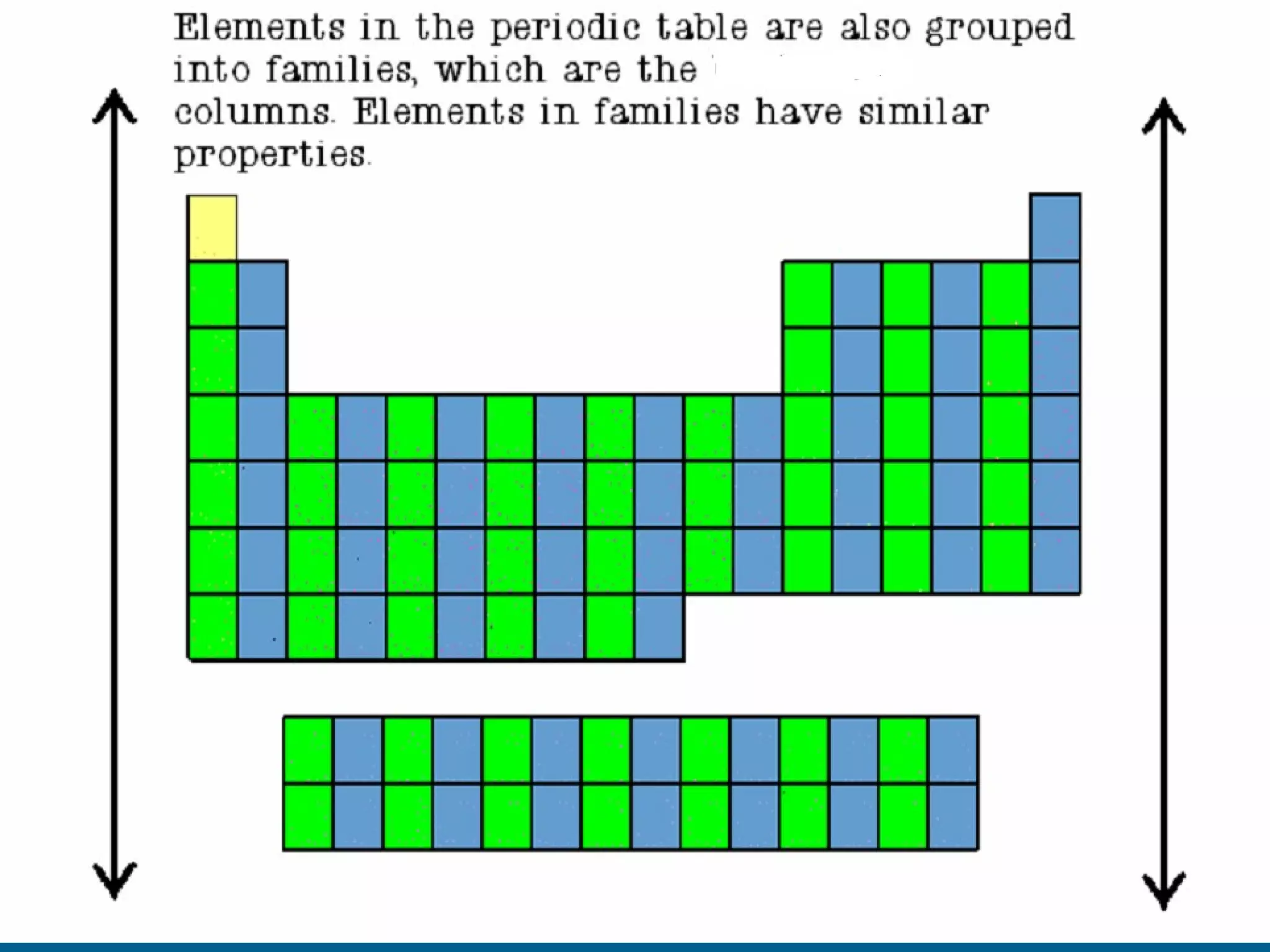
The document provides information about the periodic table of elements:
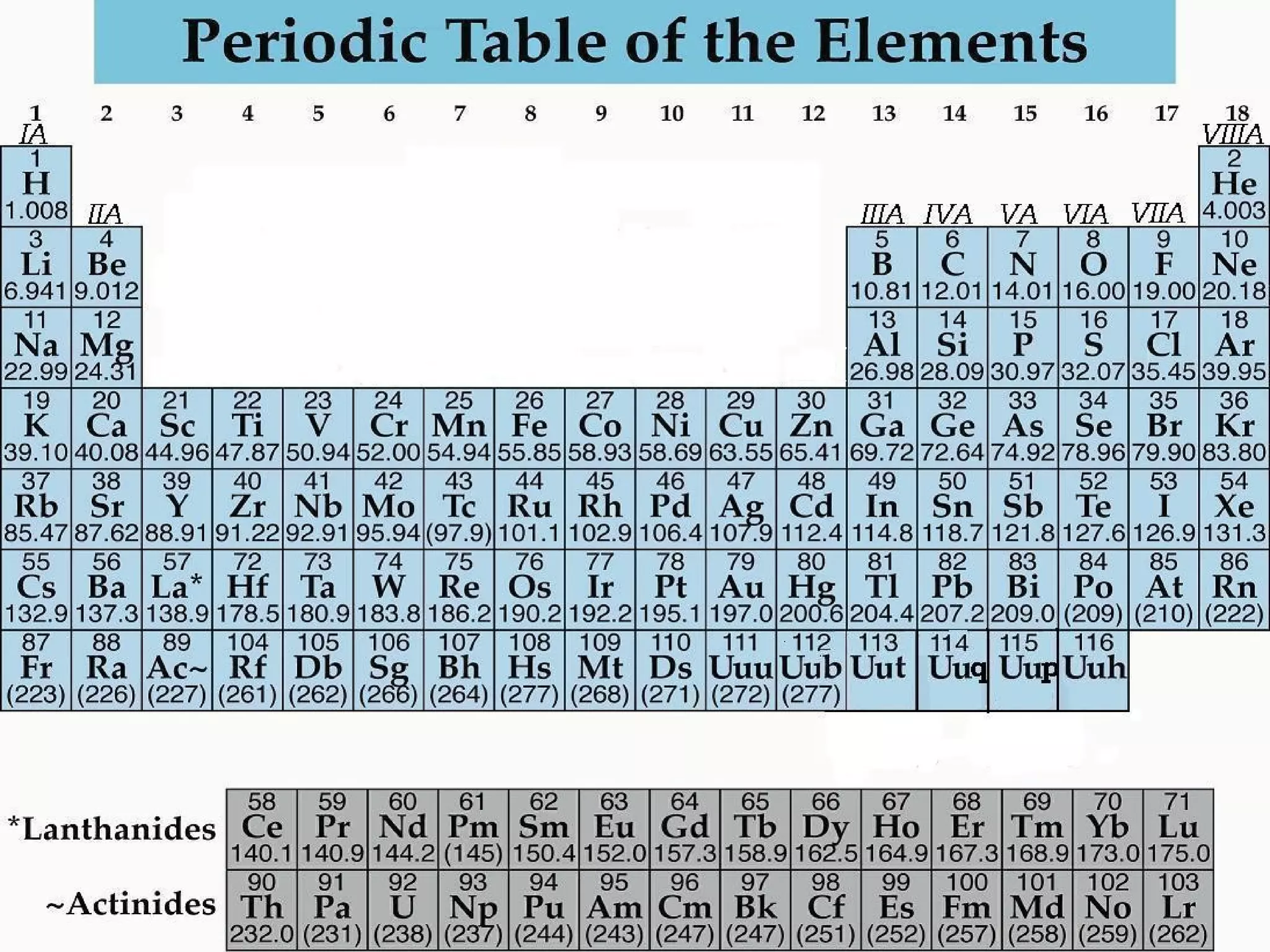
- The periodic table organizes the 118 known elements according to their atomic structure and properties. It allows one to predict chemical and physical properties of elements.
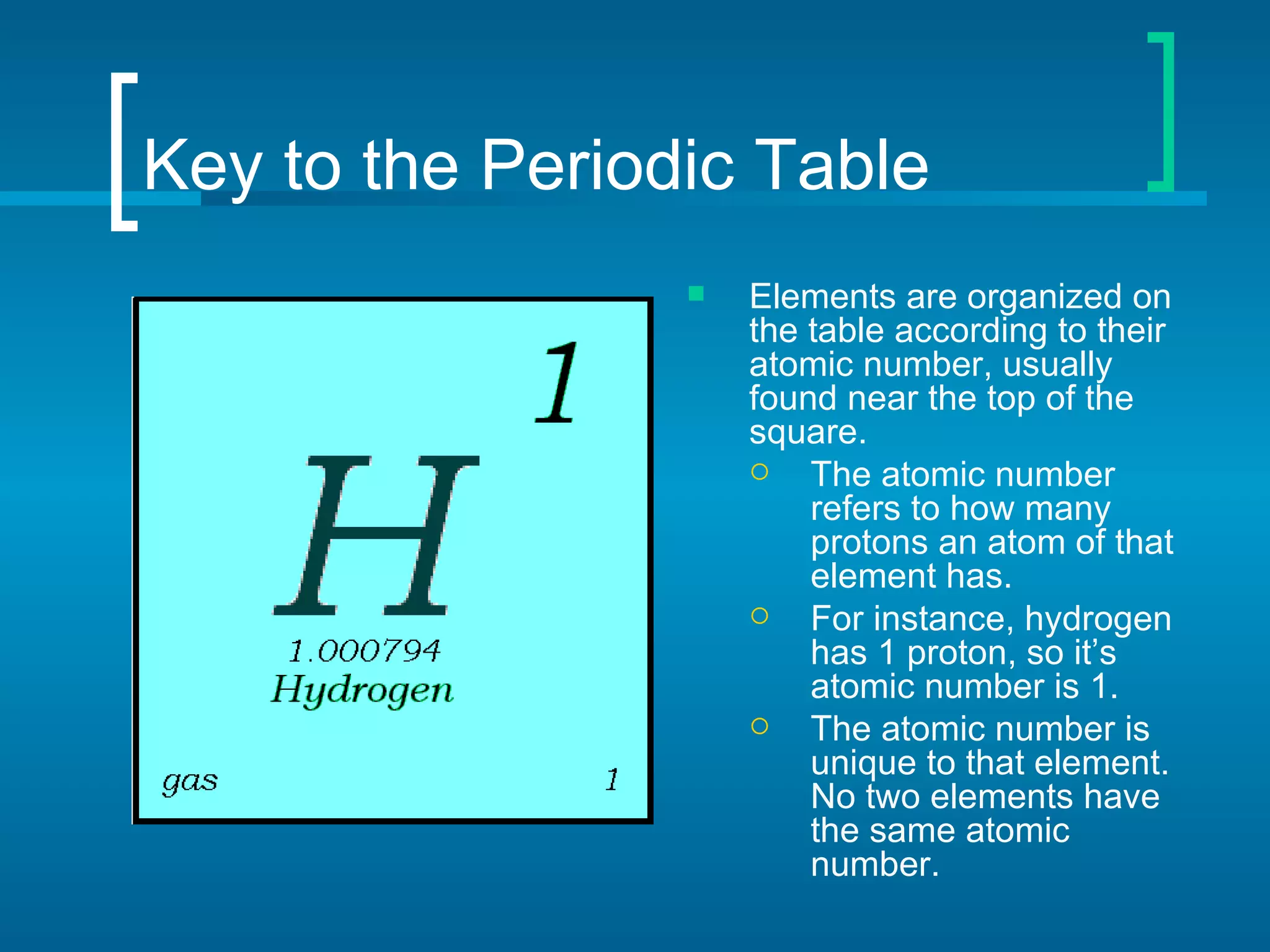
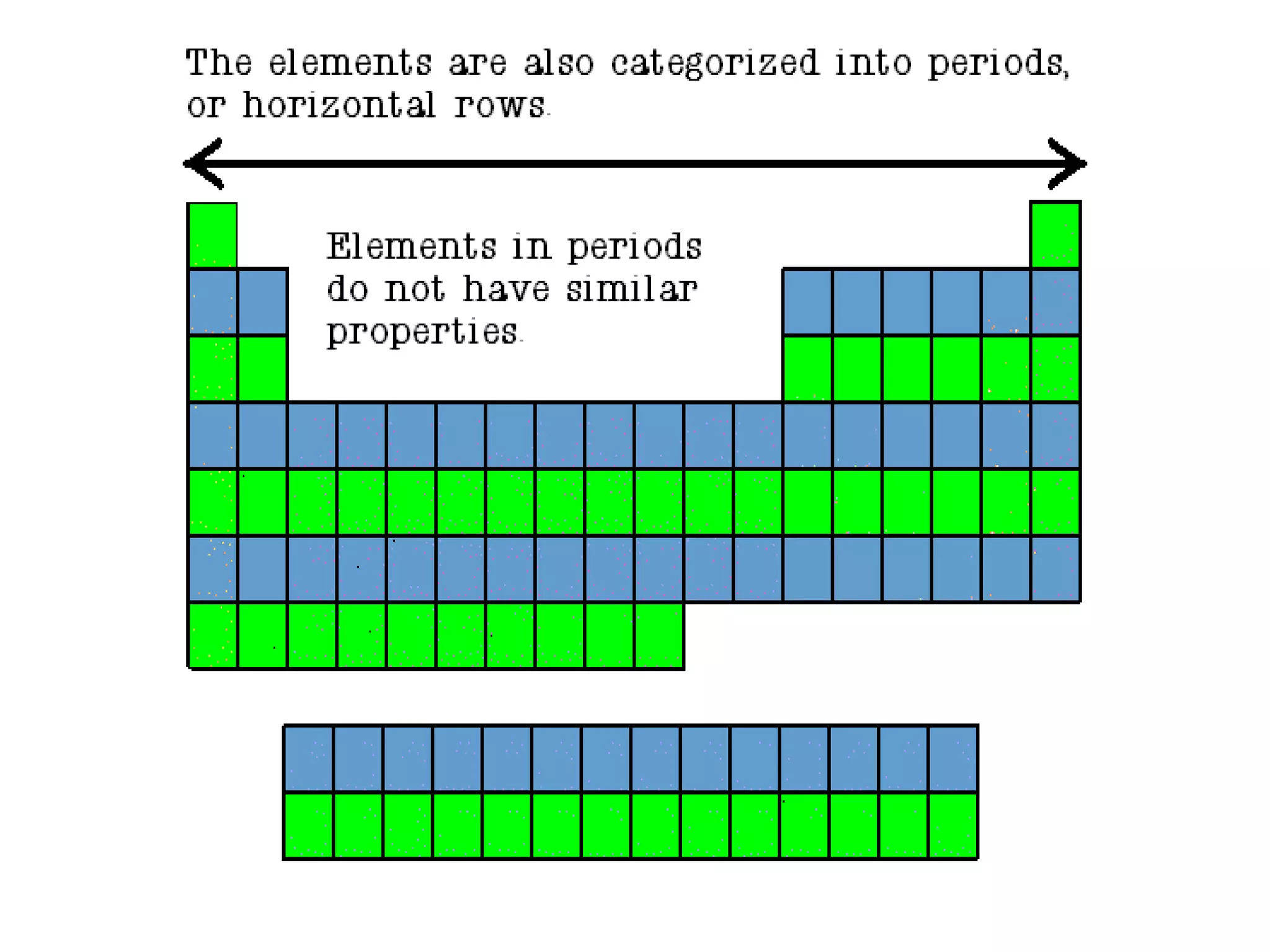
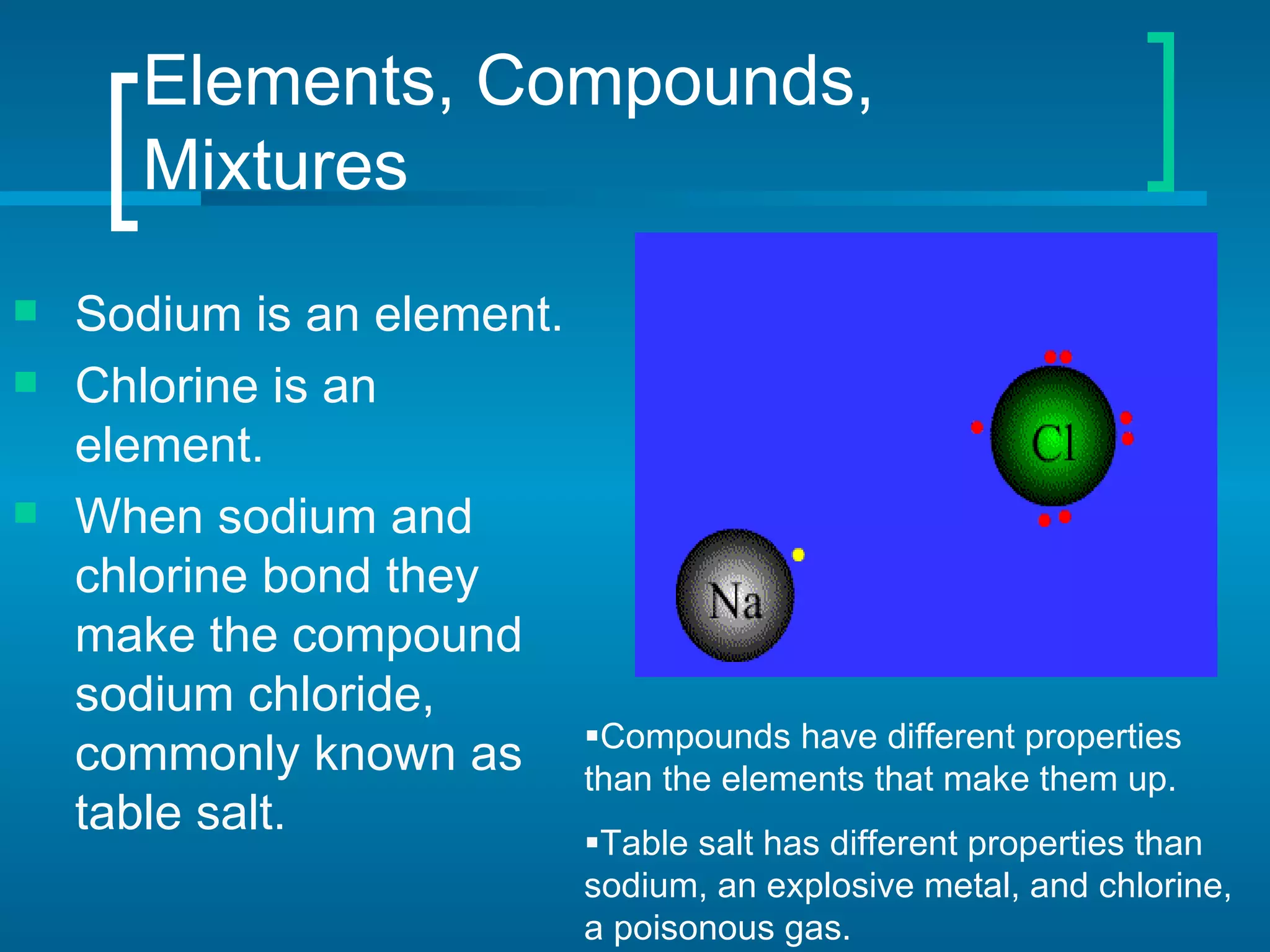
- Elements are organized by atomic number and grouped into families with similar properties. The position of an element provides information about its atomic mass, number of protons/electrons, and whether it is a metal, nonmetal, or metalloid.
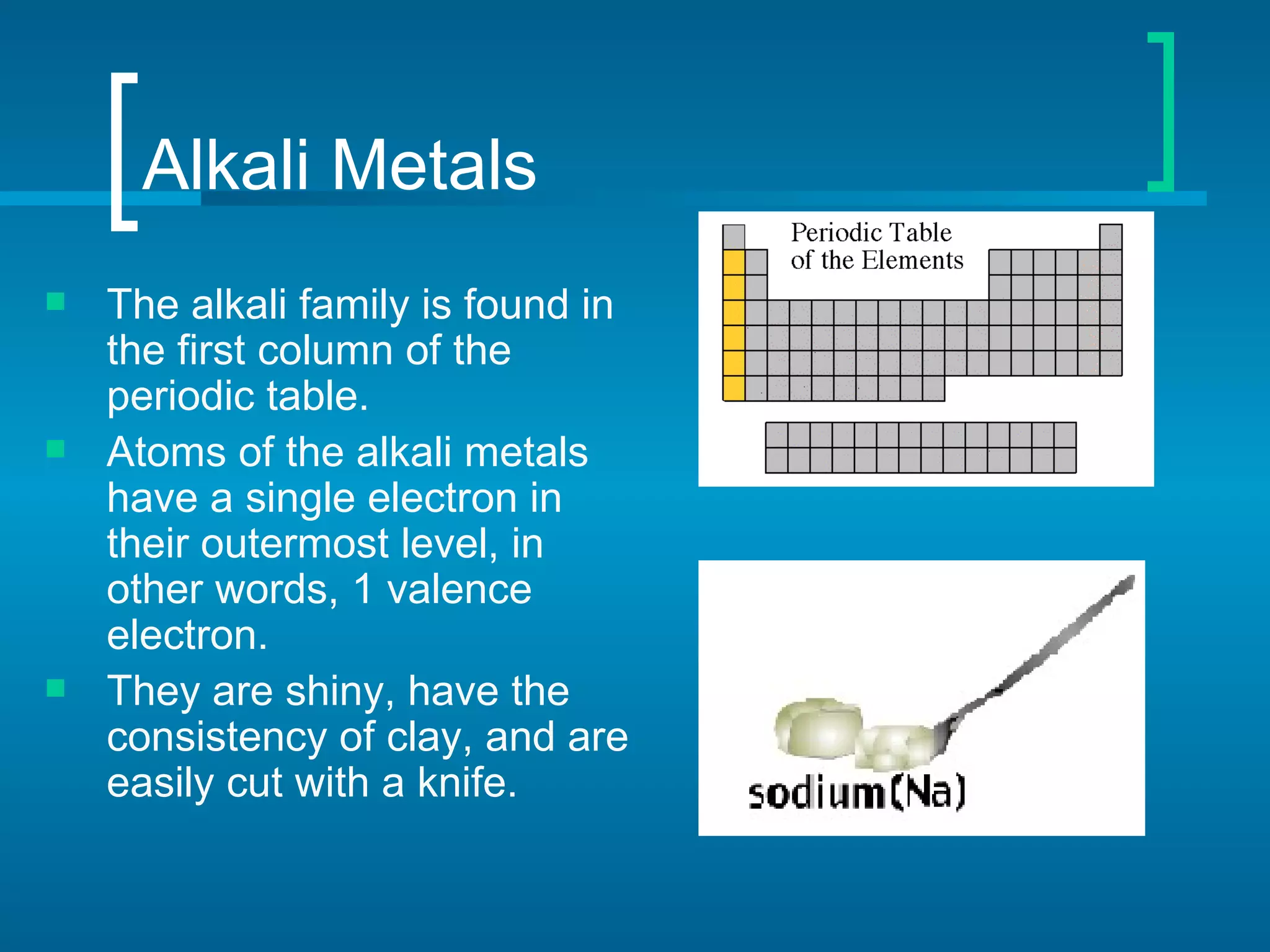
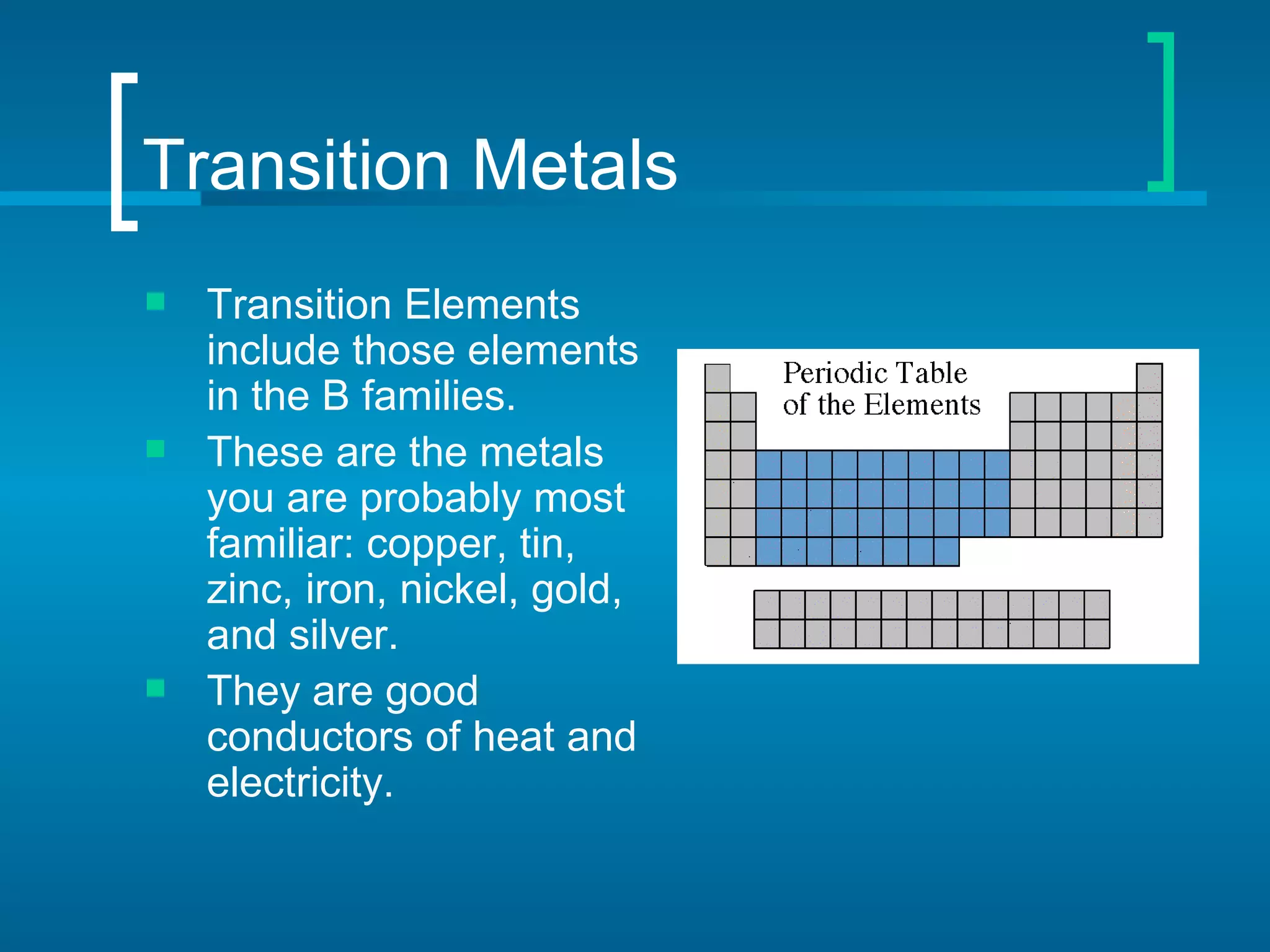
- Families include the alkali metals, alkaline earth metals, halogens, noble gases, and more. The periodic table is a useful tool for understanding elemental properties and chemical behavior.