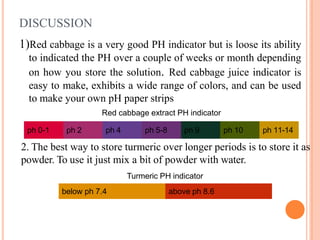
This experiment aims to make a pH meter and buffer solution. Red cabbage and turmeric extracts were tested as natural pH indicators. Red cabbage juice turns red in acid and purple/blue in neutral/base. Turmeric is yellow below pH 7.4 and pink/red above pH 8.6. Household products like vinegar, orange juice, and toothpaste were found to be acidic, acidic, and basic respectively when tested with the indicators. Both red cabbage and turmeric indicators can distinguish acid from base, with red cabbage having a wider detectable pH range.

![ABSTRACT
This experiment objective are to learn how to make pH meter and to learn
how to prepare buffer system. Acidity is measured on a pH scale. pH stands
for "potential for Hydrogen." Acidity is caused by hydrogen atoms that have
lost their electrons and are roaming free in water [H+]. The opposite of acidic
is alkaline (or basic). Alkaline gets its name from the "al kali" plant whose
ashes are capable of neutralizing acids. It does this because it contains KOH.
The "OH" is called hydroxide and as the equation shows, it combines with
the acid [H+] to form water. Extract from part of plant that have most
pigmented. Then, after filtration take place, extract of plant is used to test the
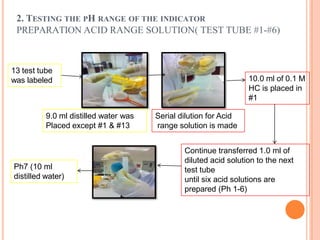
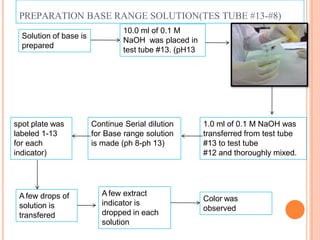
pH range of the indicator. The solution that have range 1 until 13 are
prepared to test the extract of red cabbage, turmeric, soap, detergent, vinegar
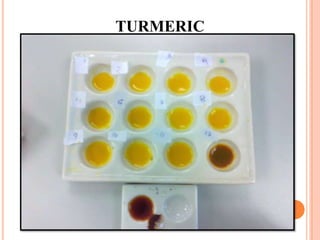
and orange juice. The pigment in red cabbage is known for its ability to
change colors depending on the pH (level of acidity) that it comes in contact
with compared to turmeric. Vinegar, orange juice. And others is needed to
check how the red cabbage and turmeric extract responds to an acid. and
based.](https://image.slidesharecdn.com/phindicator-130320204105-phpapp011-220920010638-f2b9f0ba/85/pH-indicator-experiment-2-320.jpg)