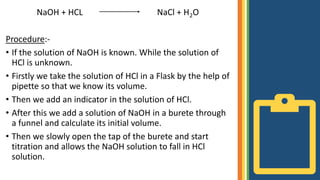
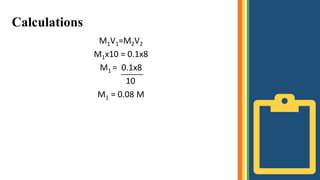
Titration is a technique used to determine the concentration of an unknown solution by using a solution of known concentration. The known solution is called the standard solution and the unknown solution is called the sample solution. Their concentrations can be calculated using the principle of M1V1=M2V2, where M1 and V1 are the molarity and volume of the unknown solution and M2 and V2 are the molarity and volume of the known solution. In acid-base titration, an acid or base of unknown concentration is neutralized by a standard solution of base or acid. Instruments like a burette, flask, stirrer, and pipette are used. The procedure involves adding the known solution from the