Embed presentation
Download to read offline





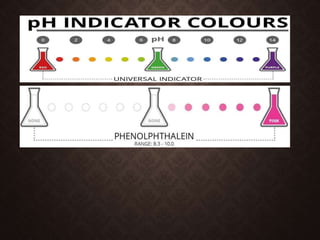
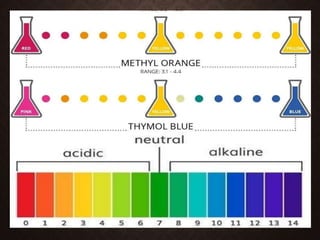
Indicators are substances that change color based on pH levels in solutions, acting as visual indicators of acidity and alkalinity. They are usually weak acids or bases whose conjugate forms have different colors, allowing the pH of a solution to be determined visually. A pH indicator detects hydronium or hydrogen ions and causes the solution's color to change based on its pH value, with values below 7.0 indicating acidity and above indicating alkalinity, while a neutral solution has a pH of 7.0.






