This document discusses various topics in nuclear and atomic physics including:
- Photon and electromagnetic radiation being quantized into particles called photons according to Einstein's theory.
- Photoelectric effect where photons eject electrons from metal surfaces.
- De Broglie hypothesis of matter waves associated with moving particles.
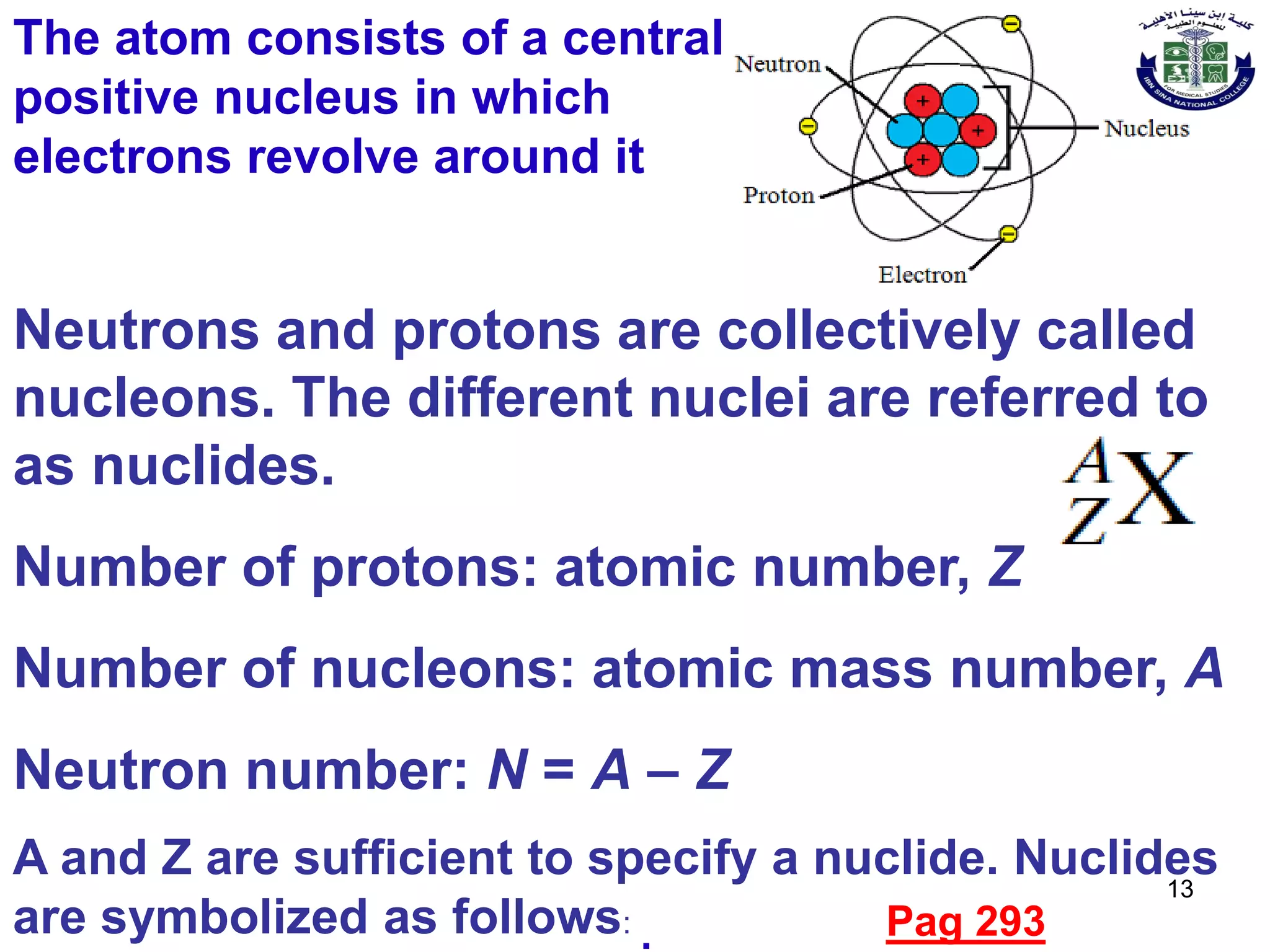
- Rutherford's model of the atom consisting of a small, dense nucleus surrounded by electrons.
- Isotopes being nuclei with the same number of protons but different numbers of neutrons.
- Nuclear radius increasing with the cube root of the atomic mass number based on measurements.
- Radioactive decay, half life, activity, and the relationship between decay constant and half life.















![Nuclear Binding Energy
The total mass ‘M’ of a nucleus is always less
than the sum of the masses (Σm) of its
separate protons and neutrons. Mass Defect,
Δ = Σm - M , Where has the mass gone?
It has become energy (E= mc2 ) [c=3x108 m/s]
Mass energy of nucleus Mc2 < total mass
energy Σmc2
This difference between these two energies is
called the total binding energy Ebe of the
nucleus.
17
Page #296
Mass Defect = Sum of masses of nucleons – mass of nucleus](https://image.slidesharecdn.com/med-181020145002/75/Med-physics-dr-ismail-atomic-and-nuclear-physics-17-2048.jpg)


![Nuclear fusion:
When two or more nuclei combine to form a
single nucleus (new element) with a
higher atomic number (more protons in the
nucleus). [Occurs in stars and sun]
Nuclear Fission: When a heavy a nucleus (ex-
U, Pu) splits into photons in the form of
gamma rays and other subatomic particles
that releases free neutrons and lighter nuclei
is called Nuclear Fission.
Page # 297](https://image.slidesharecdn.com/med-181020145002/75/Med-physics-dr-ismail-atomic-and-nuclear-physics-20-2048.jpg)
















